
Tropilaelaps clareae
Alan Wade
An emerging mite problem
What might we make of the many mites that seem never to appear on the beekeeper radar? Most are friends of the hive, in some estimates around 100 of them. As man and maid servants, most dutifully remove the insect fras and garbage that litter the floors of beehives just as does the lesser wax moth Achroia grisella. The greater wax moth, Galleria mellonella, is the great destroyer of weak hives and stored comb. Then of course there are the marauding small hive beetles, Aethina tumida and the even more feared Large African hive beetles, Oplostomus haroldi and Oplostomus fuligineus. But then what of the many parasitic, some honey bee, mites known to science? And from studies being undertaken at the Australian National University it seems there are other candidate genera of mites that may jump to honey bees signalling that we have little clue as to which pest may turn up next.
Are all Mites Almighty?
The mighty God, even the Lord, hath spoken,
And called the earth from the rising of the sun
unto the going down thereof.
Psalm 50:1
With the years 1904-1919 came a rude awakening:i the Isle of Wight (IoW) disease appeared and devastated bees across Europe. For many years the disease was attributed to the mite Acarapis woodi but that pest was not discovered and described until 1921. The mite invades honey bee trachea, their breathing tubes.
Of the fifteen known Acarapis known to parasitise invertebrates two, Acarapis dorsalis – from which Acarapis woodi seems likely to have evolved – and Acarapis externus, are cosmopolitan external mites found on both Asian and western honey bees. They are more mildly debilitating parasites than life takers.ii
It was not until 2002 that Bailey and Balliii concluded that the root cause of IoW should not be attributed to Acarapis woodi in stating that:
Conventional wisdom and beekeeping text books soon accepted that this impressive mite was the cause of the ‘Isle of Wight Disease’, yet close examination of the original paper shows that this could not be so. Rennie (and coworkers’) experimental resultsiv clearly demonstrated that some bees heavily infested with the mite were able to fly normally, yet other crawling bees, exhibiting the symptoms of the disease, contained no mites.
Neuman and Carreckv provide further clarification in stating that we are:
…led to the conclusion that the disease had been due to a combination of factors, in particular, infection by chronic bee paralysis virus (completely unknown at the time), together with poor weather which inhibited foraging, and an excess of bee colonies being kept for the amount of forage available.
A clear case of Correlation does not imply causation! Brian Johnson sensibly concludes:vi
These mites are not considered a current threat to beekeeping.
But might other mites bring our honeybees to their knees? Several phoretic mites make a limited meal of their native hosts having coevolved with them. In various measure the bees have long invented a range of defence mechanisms: giveaway odours that cause the bees to uncap and remove infected brood and mites, nipping behaviours that dismember mites, and grooming behaviours to brush off mites. There are, however, more subtle barriers to parasites jumping species and most particularly between Apis subgenera, the giant Megapis, cavity dwelling Apis and dwarf Micrapis honey bees.
By and large mites have stayed with their native hosts or migrated to species well adapted to them (Table 1). Independent of the eleven or so Asian honey bees, Apis mellifera evolved in the absence of parasitic mites (Acarapis speciesexcepted), and had developed no defence mechanisms and were not prepared for the rare event where a new parasite might arrive and thrive. Time and distance from parasitic-mite-bearing-bees meant that Apis mellifera bees, until the last several hundred years, were unlikely ever to encounter Asian honey bee mites.
| Lesser mites | Original host | Notes |
| Acarapis dorsalis | Apis cerana? | Also hosted by Apis dorsata and Apis mellifera |
| Acarapis externus | Apis cerana? | Also hosted by Apis dorsata and Apis mellifera |
| Acarapis woodi | Apis mellifera (presumptive host) | Also hosted by Apis cerana |
| Euvarroa sinhai | Apis florea | Has moved to Apis andreniformis. Also found in co-located Apis mellifera colonies where is does not persist |
| Euvarroa wongsirii | Apis andreniformis | No record of species movement |
| Leptus ariel | Free living parasitevii | Parasitises Africanised honey bees in Guatemalaviii. |
| Leptus spp. | Free living parasites | Undescribed Leptus species parasitising Apis mellifera in Brazilix |
| Leptus monteithi | Free living parasite | Parasitises colletid bee Leioproctus sp. bees in Tasmaniax |
| Leptus sp. | Stingless bees (Meliponini) | Schwarziana quadripunctata, Trigona spinipes, Melipona torrida, Scaptotrigona depilis in Argentinaxi |
| Tropilaelaps koenigerum | Apis dorsata | No record of species movement |
| Tropilaelaps thaii | Apis laboriosa | No record of species movement |
| Varroa rindereri | Apis koschevnikovi | No record of species movement |
| Varroa underwoodi | Apis cerana | No record of species movement |
Table 1 Lesser known bee mites.
Apis mellifera in Asia
Several mites have now proved to be a different kettle of fish, that is when western honey bees were moved to south east Asia and the Russian far east. Amongst the many parasitic mites two Varroa species and two species of Tropilaelaps are proving to be truly problematic (Table 2).
Let’s track back a little: Over a period of millions of years mites of the Apis cerana and the giant Apis dorsata groups remained with their native hosts and seemed unlikely to bother recently co-located regular honey bee colonies. Then very suddenly mites jumped onto our honey bees and became a new and major threat to global apiculture.
| Destructive honey bee mites | Original hosts | Notes |
| Varroa destructor | Apis cerana | Korean and Japanese halotypes only |
| Varroa jacobsoni | Apis cerana | Also hosted by Apis dorsata |
| Tropilaelaps mercedesae | Apis dorsata and Apis laboriosa | Also hosted by Apis cerana |
| Tropilaelaps clareae | Apis dorsata |
Table 2 Destructive honey bee mites, all destructive parasites of Apis mellifera
Australia has been the last great continent to fall victim to such honey bee mites. First off the rank was Varroa destructor. It was discovered well established around the New South Wales port of Newcastle in June 2022. By late February 2024 a new report of Varroa jacobsoni in Brisbane hit the airwaves.xii Previously discovered in Townsville in 2016 it was declared eradicated in 2021. This mite has devastated Apis mellifera colonies in the PNG Highlands, just as Varroa destructor has done worldwide. One might have to conclude that these mites have taken bees and beekeepers to be suckers.
Where the bee sucks, there suck I:
In a cowslip’s bell I lie;
There I couch when owls do cry.
On the bat’s back I do fly
After summer merrily.
Merrily, merrily shall I live now
Under the blossom that hangs on the bough.
Ariel’s Song, The Tempest
William Shakespeare
Are other mites likely to become a problem?
It is really not possible to predict with certainly whether any other mites might blight honey bees or indeed the whole honey bee Apis genus. Some mite species – those on bees with very restricted geographical distribution – islands, jungles, isolated mountain tops – present limited potential for transmission to Apis mellifera. Let us review the present battery of known parasitic mite genera and hazard a guess as to which ones might become a serious problem.
Acarapis mites
According to Ahn and coworkers:xiii
Acarapis mites, including Acarapis woodi, Acarapis externus, and Acarapis dorsalis, are parasites of bees [of] which [only Acarapis woodi] can cause severe damage to the bee industry by destroying colonies and decreasing honey production. All three species are prevalent throughout many countries including UK, USA, Iran, Turkey, China, and Japan.
Acarapis woodi restricts oxygen exchange in thoracic trachea and transmits viruses and bacteria.xiv It was originally reported from Europe: Scotland, France, Spain, and Greece and the United Kingdom; but then spread to the American continent, first to Argentina, Colombia, Mexico, and then the United States as well as beyond Europe to Iran, Turkey, China, and Japan. While the parasite is now widespread and has long been known to have some effect on honey beesxv, the bees are well adapted to its presence and is no longer regarded as a threat to honey bees. It has not been found in Australia.
Acarapis dorsalis has been reported from Europe, Canada, USA, New Zealand, Australia, Papua New Guinea, and Hawaii. The distribution of Acarapis externus is the similar to that of A. dorsalis, but is more abundant. Neither Acarapis externus nor Acarapis dorsalis cause much colony damage though Acarapis externus, attaching to the head and thorax of bees, has been reported to cause some wing damage.
Varroa mites
One of the problems of viewing the risk that Varroa presents to Western honey bees is that we are totally focussed on their impact on our farmed Apis mellifera bees. We tend to ignore the impact of these and other parasites actually have on their original hosts. Chiu and coworkersxvi – examining the Japanese and Korean halotypes of Varroa destructorxvii affecting western honey bees – found that that these parasite strains are hybridising with other mite halotypes through the agency of their native hosts Apis cerana. They surmise:
Previous studies indicated that Varroa mites have geographic and host-specific mitochondrial haplotypes (mitotypes) in A. mellifera colonies, suggesting that only a few genetic strains (i.e., Korean and Japanese mitotypes) can effectively infest A. mellifera. However, growing evidence indicates that native mite populations of A. cerana can hybridize with the mites imported with A. mellifera colonies. This raises the possibility for continuous host shifts of Varroa mites and the consequential hybridizations among mites from different localities, leading to erasure of host specificity among mites of different mitotypes.
Conversely mites found parasitising Apis mellifera, that is both Varroa destructor and Varroa jacobsoni, were tested by Wang and coworkers for infectivity in six populations of Apis cerana in China and Thailand.xviii Amongst the 185 colonies studies they found that the infectivity (crossover of Varroa from Apis mellifera back to Apis cerana) was similar and low. We also know that Varroa is only raised on drone brood in Apis cerana whereas it reproduces on both worker and drone brood in its unadapted host Apis mellifera. Mite reproduction is therefore strictly limited to periods when drone brood is present on its original host. Further we know that Varroa affects Apis mellifera colonies in different measure around the globe, Neumann and Carreckxix plotting its impact (Figure 1).
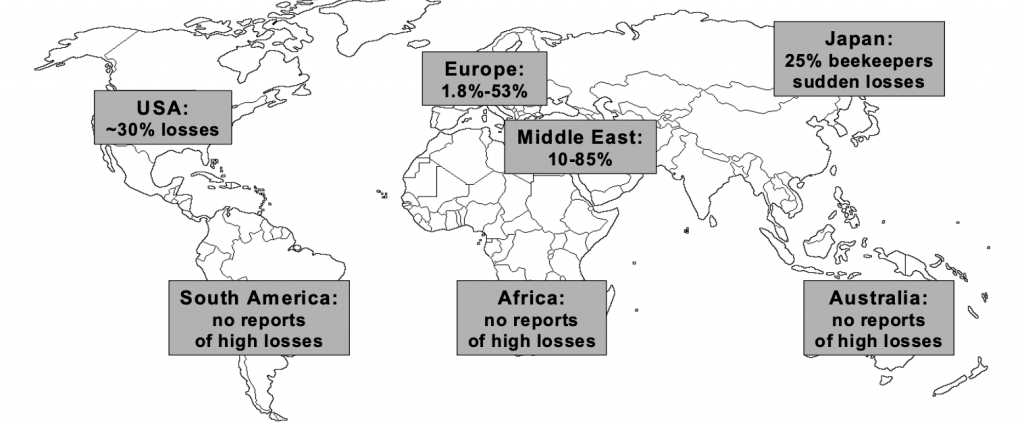
Figure 1 Global impact of Varroa as at 2010.
What about other species of Varroa?
Anderson has shown thatVarroa jacobsoni has similarly decimated Apis mellifera colonies in the PNG highlands. Although seemingly more restricted than Varroa destructor, Varroa jacobsoni poses an existential risk to our honey bees. [Here it is worth noting that Varroa destructor was originally confused with Varroa jacobsoni. Varroa jacobsoni was soon recognised to represent more than one species, the more widespread Varroa destructor and the geographically separate Varroa jacobsoni. Varroa jacobsoni also jumped to Apis mellifera from Apis cerana japonica.]
Varroa underwoodi naturally hosted by Apis cerana and Apis nigrocincta, originally confined to Indonesia and Papua New Guinea, is now widespread in populations of Apis cerana. Ilyasov and coworkersxx reports that Varroa underwoodi has spread north to Nepal, South Korea, Japan, Malaysia, India, Indonesia, Papua New Guinea, Vietnam, China and from there to the Primorsky Krai region of eastern Russia,xxi its halotype being found to be identical with that found in China. The mite has been found in Apis mellifera colonies in PNG but s not to have established itself as a pest species. Its potential threat to global apiculture is at best latent.
A further species Varroa rindereri hosted by Apis koschevnikovi, native to the Malaysian Peninsula, Sumatra, Java, Kalimantan and the Indonesian Archipelago, is now also found on Apis cerana in Irian Jaya and Papua New Guinea but has not been reported to parasitise Apis mellifera.
The mite situation is further complicated by the fact Varroa species host and transmit a range of viruses many of which many of which may have retained their virulence as a result of widespread use of arachnicides. We now discover that poor nutrition and lowered bee virus immunity, arising out of use of hard chemicals, is exacerbating what was a problem for bees at the outset.xxii In a UK study, Varroa destructor was shown to amplify cloudy wing virus, slow paralysis virus and deformed wing virus in Apis mellifera, their levels way exceeding those found prior to the arrival of the mite.xxiii
Breeding of Varroa tolerant bees is emerging as perhaps the only long term solution.xxiv

Larvae of the mite Leptus sp. parasitising Africanized honey bee Apis mellifera scutellata. Brazil, São Paulo
Photo: Erica W. Teixeira, Universidade Federal de Viçosa
So far we have covered the known parasitic mites of honey bees. We discovered that one, the tracheal mite, appears to have Apis mellifera as its native host. It is now believed to do little damage. Another,
Varroa destructor, after jumping from the Asian Honey Bee has wreaked untold carnage. Here we
extend the range of other known honey bee mites to include those with the potential to scuttle our honey bees.
Euvarroa mites
Euvarroa mites, parasites of the dwarf honey bees, differ morphologically from the Varroa mite complex mainly in respect of the structure of their respiratory systems.xxv Suffice to say the mites are physically similar and both belong to the same family Varroidae. Delfinado and Baker describe Euvarroa as parasites of dwarf honey bees in Southeast Asia, India, Korea, Japan, and the Soviet Far East.xxvi
Warrit and Lekprayoon describe the shared biology of Euvarroa and Varroa:xxvii
The mites are only capable of reproducing in the drone brood cells of their hosts and disperse to other colonies via both drones and worker bees. As with Varroa, Euvarroa causes little damage to their endemic host colonies. This may be because of the intensive grooming behaviour of the worker bees and the seasonal presence of drone brood cells that reduces the population of the mite to a minimum.
Nevertheless Euvarroa sinhai, first identified by Delfinado and Baker from samples collected in India in 1971,xxviii has been observed to reproduce in worker brood cells of Apis mellifera and survive on adult workers in colonies first treated to reduce Varroa mite levels. According to Mossadeghxxix, the life cycle of Euvarroa sinhai on Apis mellifera mirrors that of Varroa.
Other experimentation has demonstrated that while Euvarroa sinhai can survive on caged workers of Apis mellifera, Apis cerana and Apis florea there appears to be no evidence that it has become a pest of these honey bees, Guzman and coworkers noting that:xxx
Experiments under more natural conditions are required to further explore the question of whether or not Euvarroa sinhai must be considered as a new candidate for parasitism on the Western honeybee Apis mellifera.
They further note that:
Whether or not adult worker bees of different honeybee species can serve as host for Euvarroa sinhai is unknown.
However neither Asian honey bee Apis cerana nor the giant honey bee Apis dorsata, both living in close proximity (sympatry) to Apis florea, have been found to host Euvarroa sinhai. Presumably giant and Asian honey bee hygienic grooming behaviour and their propensity to swarm or migrate – behaviours they employ respectively to control Varroa and Tropilaelaps – has limited their potential to host Euvarroa though there are likely also genetic barriers to being able to do so.
Any risk to other honey bees posed by Euvarroa wongsirii is similarly difficult to anticipate. Its natural host is Apis florea’s sister dwarf honey bee, Apis andreniformis, a more recluse species. Although Apis andreniformis is sympatric with Apis florea over much of its range it has not been reported to host Euvarroa sinhai. Apis florea hosts both Euvarroa species.
Euvarroa wongsirii was first found on Apis andreniformis in western central Thailand from bees collected in 1990 and identified in 1991.xxxi Euvarroa wongsirii is now known to also parasitise Apis florea though not other honey bees. Since both Apis florea and Apis andreniformis are widespread and are sympatric over much of their range (Figure 2),xxxii the potential for both Euvarroa species to move to Apis mellifera, though seemingly unlikely, remains a possibility.
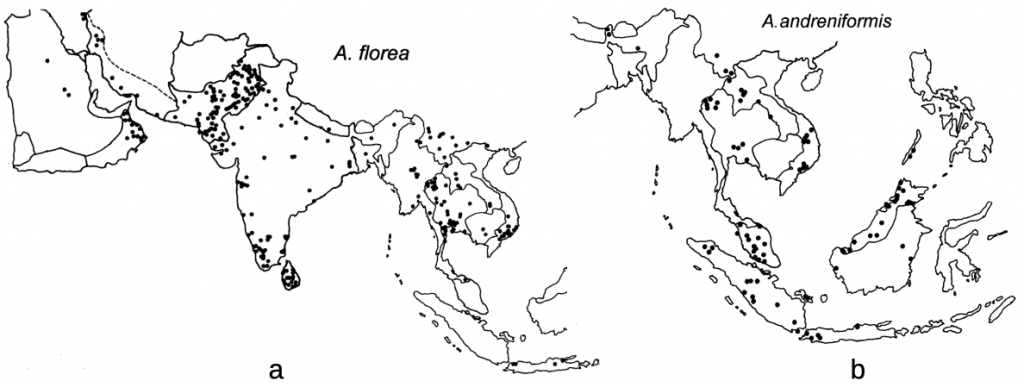
Figure 2 Distribution of dwarf honey bees and likely their respective Euvarroa species:
(a) westerly Apis florea (also now in Sudan, the Nile River Delta and Western Australia; and
(b) the more eastery Apis andreniformis in Asia.
Tropilaelaps mites
Anderson and Morganxxxiii signal that there are at least four species of this mite. They summarise what is known of their origins, natural distribution and spread. The discovery of Tropilaelaps mites and their infestation of Western honey bees comes from the Philippines. First found by a Mr G Panga, an entomologist with the Philippine Bureau of Plant Industry, they were then described by Delfinado and Baker and briefly reported on by Michael from the United States Department of Agricuture.xxxiv Tropilaelaps (tropi) mites may well eclipse Varroa mites in their impact on the Western Honey Bee. Stuart Roberts reporting on finding Tropilaelaps in Russia in March 2023xxxv records a Russian colleague stating:
Considering that Varroa also went to Europe through our country [Russia] and that the rate of spread of Tropilaelaps may be faster, it will probably reach Europe withing 3-5 years.
As to how the present spread of ‘tropi’ mites to Uzbekistan and Russia in eastern Europe will progress is uncertain. Both Tropilaelaps mercedesae and Tropilaelaps clareae parasitise the Western honey bee. Anderson and Morgan state that the present species of concern globally is emphatically Tropilaelaps mercedesae:
Tropilaelaps clareae, previously assumed to be ubiquitous in Asia, was found to be two species, and it is here redefined as encompassing haplotypes (mites with distinct mtDNA gene sequences) that parasitise native Apis breviligula and introduced Apis mellifera in the Philippines and also native Apis dorsata binghami on Sulawesi Island in Indonesia.
The now rampant Tropilaelaps mercedesae hosted naturally by Apis dorsata is now also a parasite of Apis cerana, Apis florea and Apis mellifera.
Tropilaelaps mercedesae together with Tropilaelaps koenigerum parasitise native Apis dorsata in mainland Asia and Indonesia (except Sulawesi Island) while Tropilaelaps mercedesae also parasitises introduced Apis mellifera in these regions.
Tropilaelaps koenigerum encompasses haplotypes that parasitise and reproduce on Apis dorsata in Sri Lanka, mainland Asia and Indonesia (except Sulawesi Island).
Tropilaelaps thaii parasitises Apis laboriosa in mountainous Himalayan regions. Tropilaelaps thaii is now widely distributed in South and Southeast Asia and is found mainly in forested areas: Terai of Nepal, Malaysia and Singapore. Too little is known of Tropilaelaps koenigerum and Tropilaelaps thaii to know whether they, too, are a threat to the Western (Apis mellifera) and Asian (Apis cerana group) of honey bees
Leptus mites
While these distinctively red mites are a seeming novelty to beekeeping, there is little reason to believe they will exert much influence on honey bees except perhaps in the central and southern tropical Americas. Reporting of their incidence in Apis mellifera colonies is sparse, most being published in Spanish and Portuguese.
The relatively recent discovery of the Leptus ariel parasitising Africanised honey bees may come as a surprise to many beekeepers. There are of the order of 150 species of Leptus that parasitise a wide range of adult invertebrates, but only a few have been found on hymenopteran ants, wasps and bees. The level of infestation of honey and stingless bee hives with Leptus species is generally low (typically less than 10%), previously infected colonies often being free of the mite.
Ronald Southcott, partly famous for his study of the box jellyfish Chironex fleckeri, is principally recognised for his pioneering work unravelling the taxonomy of scorpions, ticks and mites, notably the complex life cycles of Acari mites including those of the genus Leptus. Several species, including Leptus ariel, parasitise adult Africanised honey bees. These have been recorded by Martin and Correia-Oliveira for Leptus ariel in Guatemala as well as by Teixeira for a Leptus species Brazil but their occurrence in neighbouring countries is unknown.
Where go the mighty mites
Future mite incursions to Australia appear almost inevitable, while more mites of concern will come onto the global beekeeping scene. Tropilaelaps will make its mark and more than one species of Varroa will likely become problematic. Eventually Varroa and other mites will evolve and their impact will moderate, that is if beekeeper intervention designed to fully control mites does not prevent this happening.
The nightmarish scenario where both Varroa and Tropilaelaps arrive and compete one with the other signals that we will need strategies to combat mites on many fronts. Several mites, Varroa jacobsoni, Tropilaelaps mercedesae and Tropilaelaps clareae are one flight or cargo ship away from south east asian countries and Pacific nations including Papua New Guinea, so represent a genuine and existential threat to Australian honey bees.
Readings
iCushman, D.A. (2018). Isle of Wight (IoW) disease: Was it really as we are led to believe? http://www.dave-cushman.net/bee/iowdisease.html
iiSammataro, D., Gerson, U. and Needham, G. (2000). Parasitic mites of honey bees: Life history, implications, and impact. Annual Review of Entomology 45(1):519-548. https://pubmed.ncbi.nlm.nih.gov/10761588/
iiiBailey, L. (2002) The Isle of Wight disease. Central Association of Bee-Keepers; Poole, UK. 11 pp.
Bailey, L. and Ball, B.V. (1991). Honey bee pathology, Chapter 7, Parasitic mites pp.78-93. Academic Press, London, UK. 193pp.
ivRennie, J., White, P.B. and Harvey, E.J. (1921). Isle of Wight Disease in hive bees. Transactions of the Royal Society of Edinburgh 52:737-779. https://ia601305.us.archive.org/5/items/cu31924003692633/cu31924003692633.pdf
v Neumann, P. and Carreck, N. (2010). Honey bee colony losses. Journal of Apicultural Research 49(1):1-6. doi:10.3896/IBRA.1.49.1.01
viJohnson, B.R. (2023). Honey Bee Biology. Princeton University Press.
viiMartin, S.J. and Correia-Oliveira, M.E. (2016). The occurrence of ecto-parasitic Leptus sp. mites on Africanized honey bees. Journal of Apicultural Research 55(3):243-246. doi:10.1080/00218839.2016.1228214
viiiSouthcott, R.V. (1989). A larval mite (Acarina: Erythraeidae) parasitizing the European honey bee in Guatemala. Acarologia 30(2):123-129. https://www.semanticscholar.org/paper/A-larval-mite-(Acarina%3A-Erythraeidae)-parasitizing-Southcott/3953661ef6c557cda25e3234d945334f189719fc?sort=relevance&page=2 https://www1.montpellier.inra.fr/CBGP/acarologia/article.php?id=2522
ixTeixeira, É.W. (2008). Report on the occurrence of Leptus sp. Latreille 1796 (Acarina: Erythraeidae) on africanized honey bees A. mellifera Linnaeus 1758 (Hymenoptera: Apidae) workers, in Brazil. [Ocorrência de larvas de Leptus sp. Latreille 1796 (Acarina: Erythraeidae) em operárias de abelhas africanizadas A. mellifera Linnaeus 1758 (Hymenoptera: Apidae), no Brasil.] Boletim de Indústria Animal 65(3):249-251. https://www.semanticscholar.org/paper/Ocorr%C3%AAncia-de-larvas-de-Leptus-sp.-Latreille-1796-Teixeira/922690cfea51635cc3998bd4882033d23222bbed http://bia.iz.sp.gov.br/index.php/bia/article/view/1130/1124
xSouthcott, R.V. (1993). Larvae of Leptus (Acarina: Erythraeidae) ectoparasitic on higher insects of Australia and New Guinea. Invertebrate Taxonomy 7(6):1473-1550. https://www.publish.csiro.au/IS/IT9931473
xiMartínez, P.A, Alvarez, L.J. Garrido, P.M., Porrini, D.P., Muller, P.F., Alberoni, D. and Porrini, M.P. (2023). First record of Leptus spp.(Acari: Erythraeidae) parasitizing stingless bees (Apidae: Meliponini). Journal of Apicultural Research 62(4):6pp. https://ri.conicet.gov.ar/bitstream/handle/11336/223390/CONICET_Digital_Nro.7db2aba4-3d8b-47ff-89a5-38c84c10202e_E.pdf?sequence=5
xiiHughes, M. (22 February 2024). Biosecurity Queensland investigating detection of Varroa jacobsoni mite at the Port of Brisbane. https://www.abc.net.au/news/2024-02-26/varroa-jacobsoni-mite-detected-in-port-of-brisbane/103512374
xiiiAhn, A.-J., Ahn, K.-S., Noh, J.-H., Kim, Y.-H., Yoo, M.-S., Kang, S.-W., Yu, D.-H. and Shin, S.S. (2015). Molecular prevalence of Acarapis mite infestations in honey bees in Korea. Korean Journal of Parasitology 53(3):315-320. doi:10.3347/kjp.2015.53.3.315 https://www.cabidigitallibrary.org/doi/pdf/10.5555/20153364149
xivSammataro, D., Gerson, U. and Needham, G. (2000) loc. cit.
xvRennie, J.; White, P.B. and Harvey, E.J. (1921) loc. cit.
xviChiu, Y.F., Nguyen, T.T.H., Yeh, P.T., Cronin, A.L., Peng, P. and Su, Y.C. (2023). Genome-wide SNPs show hybridization of Varroa mites from different Apis hosts in Vietnam and Taiwan. Apidologie 54(2):25-. https://www.researchgate.net/publication/369777773_Genome-wide_SNPs_show_hybridization_of_Varroa_mites_from_different_Apis_hosts_in_Vietnam_and_Taiwan
xviiSolignac, M., Cornuet, J.M., Vautrin, D., Le Conte, Y., Anderson, D., Evans, J., Cros-Arteil, S. and Navajas, M. (2005). The invasive Korea and Japan types of Varroa destructor, ectoparasitic mites of the Western honeybee (Apis mellifera), are two partly isolated clones. Proceedings of the Royal Society B: Biological Sciences 272(1561):411-419. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1634981/ doi:10.1098/rspb.2004.2853
xviiiWang, S., Lin, Z., Chen, G., Page, P., Hu, F., Niu, Q., Su, X., Chantawannakul, P., Neumann, P., Zheng, H. and Dietemann, V. (2020). Reproduction of ectoparasitic mites in a coevolved system: Varroa spp. – Eastern honey bees, Apis cerana. Ecology and Evolution ece3.7038–. https://onlinelibrary.wiley.com/doi/10.1002/ece3.7038 doi:10.1002/ece3.7038
xixNeumann, P. and Carreck, N.L. (2010) loc. cit.
xxAnderson, D.L., Halliday, R.B. and Otis G.W. (1997). The occurrence of Varroa underwoodi (Acarina: Varroidae) in Papua New Guinea and Indonesia. Apidologie 28:143-147. https://hal.archives-ouvertes.fr/hal-00891413/document
xxiIlyasov, R.A., Takahashi, J.I., Lee, M.L., Proshchalykin, M.Y., Lelej, A.S., Kwon, H.W., Danilenko, V.N. and Nikolenko, A.G. (2022). Characteristics of Varroa underwoodi mites (Acari: Varroidae) in the population of Apis cerana ussuriensis (Hymenoptera: Apidae) in the Primorsky Krai of Russia. Biology Bulletin Reviews 12(5):516-526. https://doi.org/10.1134/S2079086422050048
Ilyasov, R.A., Takahashi, J.I., Lee, M.L., Proshchalykin, M.Y., Lelej, A.S., Kwon, H.W., Danilenko, V.N. and Nikolenko, A.G. (2022). Characteristics of Varroa underwoodi mites (Acari: Varroidae) in the population of Apis cerana ussuriensis (Hymenoptera: Apidae) in the Primorsky Krai of Russia. Журнал Общей Биологии 83(1):38-50. https://elementy.ru/genbio/abstracts/685/Characteristics_of_Varroa_underwoodi_mites_Acari_Varroidae_in_the_population_of_Apis_cerana_ussuriensis_Hymenoptera_Apidae_in_the_Primorsky_Krai_of_Russia
Ilyasov, R.A., Takahashi, J. and Proshchalykin, M. (2021). First evidence of presence of Varroa underwoodi mites on native Apis cerana colonies in Primorsky Territory of Russia based on COX1 gene. Journal of Apicultural Science 65(1):177-187. doi:10.2478/jas-2021-0014 https://www.researchgate.net/publication/352741847_First_Evidence_of_Presence_of_Varroa_underwoodi_Mites_on_Native_Apis_cerana_Colonies_in_Primorsky_Territory_of_Russia_Based_on_COX1_Gene
xxiiPenn State University (3 April 2023). Viruses in honey bees. https://extension.psu.edu/viruses-in-honey-bees/
xxiiiCarreck, N.L., Ball, B.V. and Martin, S.J. (2010). Honey bee colony collapse and changes in viral prevalence associated with Varroa destructor. Journal of Apicultural Research 49(1):93-94. https://doi.org/10.3896/IBRA.1.49.1.13
xxivRiley, S. (2024). Westerham Beekeepers The honey bee solution to Varroa: A practical guide for beekeepers. Northern Bee Books, Scout Bottom Farm, Mytholmroyd, West Yorkshire
xxvBruce, W.A., Delfinado-Baker, M. and Vincent, D.L. (1997). Comparative morphology of the peritremes of Varroa and Euvarroa (Varroidae), parasites of honey bees (Apidae). International Journal of Acarology 23(1):13-20. doi:10.1080/01647959708684114
xxviDelfinado, M.D. and Baker, E.W. (1974). Varroidae, A new family of mites on honey bees (Mesostigmata: Acarina). Journal of the Washington Academy of Sciences 64(1):4-10. http://www.jstor.org/stable/24535743.
xxviiWarrit, N. and Lekprayoon, C. Chapter 16 Asian honeybee mites. pp.347-368 in Hepburn, R. and Radloff, S.E. (2011). Honeybees of Asia. Springer, Heidelberg, Dordrecht, London, New York.
xxviiiChantawannakul, P., de Guzman, L.I., Li, J. and Williams, G.R. (2016). Parasites, pathogens, and pests of honeybees in Asia. Apidologie 47(3):301-324. doi:10.1007/s13592-015-0407-5
xxixMossadegh, M.S. (1990). Development of Euvarroa sinhai (Acarina: Mesostigmata), a parasitic mite of Apis florea, on A. mellifera worker brood. Experimental and Applied Acarology 9(1):73-78. doi.org/10.1007/BF01198984
xxxKoeniger, N., Koeniger, G., De Guzman, L. I. and Lekprayoon, C. (1993). Survival of Euvarroa sinhai Delfinado and Baker (Acari, Varroidae) on workers of Apis cerana Fabr, Apis florea Fabr and Apis mellifera L in cages. Apidologie 24(4):403-410. doi:10.1051/apido:19930407
xxxiLekprayoon, C. and Tangkanasing, P. (1991). Euvarroa wongsirii, a new species of bee mite from Thailand. International Journal of Acarology 17(4):255-258. doi:10.1080/01647959108683915 https://sci-hub.53yu.com/10.1080/01647959108683915
Wongsiri, S., Limbipichai, K., Tangkanasing, P., Mardan, M.. Rinderer, T.. Sylvester, H.A., Koeniger, G. and Otis, G. (1990). Evidence of reproductive isolation confirms that Apis andreniformis (Smith, 1858) is a separate species from sympatric Apis florea (Fabricius, 1787). Apidologie 21(1):47-52. https://www.apidologie.org/articles/apido/pdf/1990/01/Apidologie_0044-8435_1990_21_1_ART0006.pdf doi.org/10.1051/apido:19900106
xxxiiOtis, G.W. (1996). Distributions of recently recognized species of honey bees (Hymenoptera: Apidae; Apis) in Asia. Journal of Kansas Entomological Society 69(4):311-333. Supplement: Special Publication Number 2: Proceedings of the Eickwort Memorial Symposium (October 1996). https://www.jstor.org/stable/25085727 https://wgbis.ces.iisc.ac.in/biodiversity/sahyadri_enews/newsletter/issue46/bibliography/47_Distributions%20of%20recently%20recognized%20species%20of%20honey%20bees.pdf
xxxiiiAnderson, D.L. and Morgan, M.J. (2007). Genetic and morphological variation of bee-parasitic Tropilaelaps mites (Acari: Laelapidae): New and re-defined species. Experimental and Applied Acarology 43(1):1-24. doi:10.1007/s10493-007-9103-0 https://openresearch-repository.anu.edu.au/bitstream/1885/51418/2/01_Anderson_Genetic_and_morphological_2007.pdf https://pubmed.ncbi.nlm.nih.gov/17828576/
xxxivDelfinado, M.D. and Baker E.W. (1961). Tropilaelaps, a new genus of mite from the Philippines (Laelapidae [s. lat.], Acarina). Fieldiana Zoology 44(7):53-56. https://www.biodiversitylibrary.org/partpdf/36189
Michael, A. S. (1962). Tropilaelaps clareae, a mite infesting honeybee colonies. Bee World 43(3):81-82. doi:10.1080/0005772x.1962.11096944
xxxvRoberts, S. (March 2023). Newsround: Yet another pest. The Beekeepers Quarterly 151:9.
Carreck, N. (June 2023). Beyond the veil: Is Tropilaelaps on the move? The Beekeepers Quarterly 152:8-9.
