
Alan Wade
After all is said and done,
More is said than done.
Traditional aphorism
Scattered reports of varroa in the Canberra region tell us the time for talking is over. Get out there, conduct regular mite checks and kit up to treat bees. The alternative is to lose a pastime. Here are the stark realities:
- mites are here to stay and likely widespread though their numbers are presently low;
- mite surveillance has been the exception and most keepers will not realise their bees are infected until they fall off a cliff;
- mites are hard to detect at low levels while tropi mites – when they turn up – will be both harder to monitor and treat; and
- mite populations will explode in the initial pandemic wave and there will be sudden and catastrophic colony losses.
But hey, apiarists still keep bees worldwide, many doing better that others and we don’t have deformed wing virus or indeed the Tropilaelaps (tropi) mite.
Monitor, monitor, monitor
In October 2024, one of my swabbed hives returned a positive DNA varroa result. Alcohol washes conducted on 11 March and 4 April this year failed to detect any mites. So that genetic fingerprint check was a false alarm. That our bees are OK now may prove to be a very different story when we make that first inspection next spring.
Honey bee colonies produce a flush of bees in early-to-mid autumn. They are programmed to do this to sustain themselves over winter. If, perchance, these potentially long-lived (so-called diutinous) bees are weakened by parasitism and viruses, their lifespan is severely curtailed. Such colonies often fail, or nearly do so, over winter or as they attempt to raise brood coming into spring.
Time to monitor
We must however stop and ask: ‘What are the most effective means of tracking mite numbers to determine whether to intervene?’ In practice monitoring is limited to collecting mites from brood combs or recovering mites when they fall from the brood nest (Table 1).
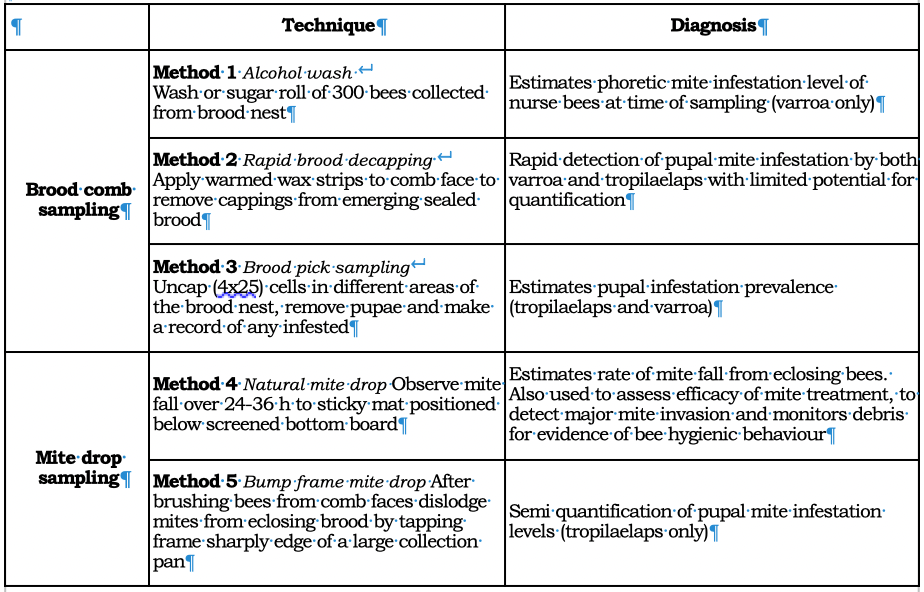
Table 1 Sampling techniques for detecting and assessing levels of mite infestation.
Varroa mites
From early spring up until the end of autumn, most mites (~80%) are cocooned below brood cappings. This means that any estimate of the total mite infestation level will be out by a factor of up to five. As brood rearing comes to a near end by late autumn, the main mite pupal food source dries up. All things being equal and with the bee population declining there will be proportionally more mites per bee. More stringent mite control is imperative just prior to this time but sampling will now provide a truer estimate of the mite infestation level.
Tropilaelaps mites
Sampling for tropi mites has proved to be exceptionally challenging. Few mites, just a couple of percent, are ever on the comb face and there are virtually none on adult bees. As the British National Bee Unit (Gill, 2024; Gill and coworkers, 2024) outlines, the options for detecting and monitoring can be boiled down to:
- brood decapping where brood is removed and examined for the presence of mites;
- the bump method where frames of brood are rapped sharply to dislodge running mites;
- floor debris sampling where debris is collected and sent off for laboratory analysis; and
- sticky floor inserts where mites drop after miticide treatment.
Brother Adam boiled bee boxes in caustic lye – sodium hydroxide – to rid his gear of American Foul Brood. Eradicating bee diseases, requeening and maintaining barriers – not swapping gear between hives and not exchanging gear between apiaries – have become essential tenets of good beekeeping practice. Regardless none of these measures cut the cake when it comes to keeping bee mites at bay. This is not to say that euthanasing collapsing colonies or controlling mite levels in neighbouring hives will not be helpful. However catastrophic mite invasions from dying colonies and rebound of mite populations are always on the card. Proactive mite control measures, though essential, will only ever offer temporary reprieve since parasitic mites have a well earned reputation of returning with a vengeance.
Given we have mites, we must ask ourselves what we really want to know about the real world condition of our hives. We certainly need to know if there are a lot of mites present, if a treatment has achieved its objective of killing mites or whether, even after a short time, colonies have relapsed. For these reasons we may not need to have a true estimate of actual mite numbers. But we do need to know whether bees are heavily infested or soon will be so.
Method 1 Alcohol wash
The standard practice is to alcohol wash mites nurse bees to measure the severity of adult bee parasitism (mites per bee). Conventional wisdom is that the threshold for treatment is reached when mite numbers reach a 2% (6 mites per half cupful of bees) infestation level irrespective of hive strength. Randy Oliver (Wade, 2025) signals that this threshold may well be too high while Russell Smith in New Zealand nominates mite thresholds of 5+ mites in autumn/spring and 9+ mites in summer. In practice this means not treating bees if there are only one or two mites in a half cup wash, routinely treating all colonies in an apiary if a handful of mites are detected or hot treating (4.2% oxalic acid w/v; Oliver, 2023) or euthanasing bees if mite numbers are way out of control.
To save time some commercial beekeepers short cut the step of measuring out the half cup of bees. Instead they simply run the lip of the test jar gently down brood comb faces so that bees drop directly into the alcohol wash. In doing this they fairly adroitly adjudge the number of bees required that is after making quite certain that the queen is not on the comb face.
In practice conducting an alcohol wash in the dead of a Canberra winter or having to remove supers in the middle of a major honey flow is not feasible. Instead the non invasive mite drop technique (Method 4) can be employed to keep an eye on the level of mite infestation.
Method 2 Rapid brood decapping
A rapid brood decapping technique, developed in response to the need for effective Tropilaelaps monitoring, is gaining considerable traction. It is proving to be particularly useful where bees are co-infected with Varroa and Tropilaelaps mites. Though mainly employed for mite detection, rapid brood decapping has much in common with the laborious brood pick sampling method (described below). That technique better quantifies the level of brood infestation.
In the rapid brood decapping procedure, warmed rectangular wax strips (Veet® Australia, 2025) are hand warmed and applied to a patch of emerging brood. This strip is then peeled off to expose a little over 200 pupae (Uzunov et al., 2025b, 2025c) (Figure 1). Once examined for the presence of fast moving tropi mites, and less mobile varroa mites, the comb is returned to the nest where nurse bees quickly recap healthy brood. Aleksandar Uzunov demonstrates the procedure in his presentation to the 22 April 2025 Apimonda Tropilaelaps webinar (time slot 1.15.39 – 1.23.59). To validate the technique, the comb face was video recorded to compare field and filmed findings.
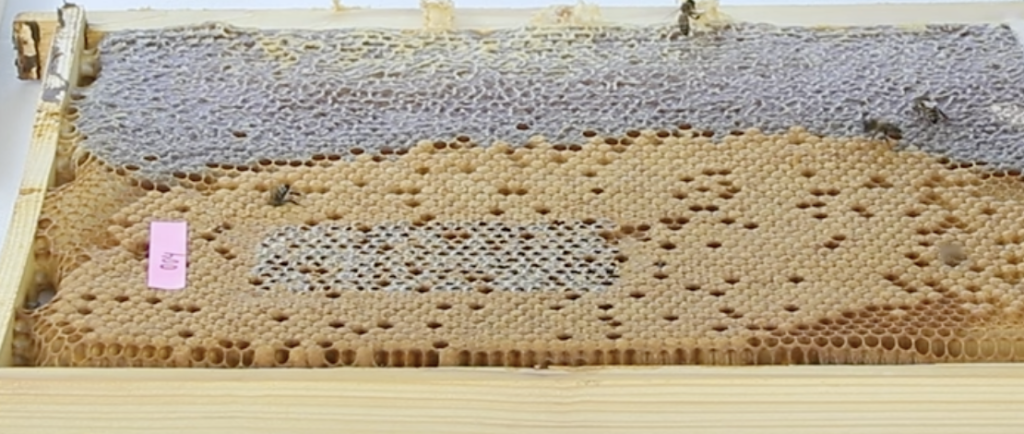
Figure 1 Rapid brood decapping showing exposed brood (lower centre) after peeling off warmed wax strip.
Source: Aleksander Uzunov Apimonda tropilaelaps webinar 22 April 2025
Method 3 Brood pick sampling
The Apimonda Tropilaelaps 22 April 2025 forum leader Jeff Pettis outlined his published method (Pettis et al., 2013) where individual brood cells are sampled across four brood comb faces, a total of 4×25 cells. Each cell is individually uncapped with tweezers and the larva is removed and checked for mites. Since tropi mites are very mobile only presence of absence of mites, not the number of mites per cell, is recorded.
Method 4 Natural mite drop
Standard methods for Varroa and Tropilaelaps mites research are outlined by the Colony Loss Honey Bee Research Association (COLOSS). Their manual, Standard methods for Apis mellifera pest and pathogen research (Dietemann et al., 2013a; Dietemann et al., 2013b; Anderson and Roberts, 2013) commends mite drop as a non invasive technique for early mite detection and for monitoring trends in mite numbers. The technique employs an oiled white landing board (e.g. corflute) or baking paper in a tray located below a screened bottom board.
The are downsides to relying on daily mite drop to monitor mite levels. The collection plate requires cleaning or replacement and requires return visits to the apiary. Mite drop is an indirect measure of the level of infestation but will signal sudden mite invasion or build up in mite numbers. Mite drop is also employed to monitor treatment efficacy and to monitor tell-tale mite hockey stick debris produced by hygienic bees. Pettis et al. (2013) report that Tropilaelaps mites, being very small, are difficult to discriminate from fallen debris, limiting its potential to monitor tropi infestation.
Method 5 Bump frame mite drop
Bumping frames to dislodge mites (Pettis et al., 2013; Ramsey, 2018) is the ‘tropi alternative’ to the alcohol wash for ‘varroa monitoring’. Brood frames, free of bees, are bumped sharply on the rim of a large collection pan. Downsides of the technique are that sharp jolting of brood may damage imago – late instar – pupae and that only a small percentage of mites will fall.
Time to treat
Estimating the size of mite populations in hives is more an art form than a scientifically objective measurement but is a useful call to initiate a brood break or hasten to treatment. Having determined that bees are heavily infested or that a threshold for treatment has been reached, we now need to choose a treatment that is effective taking into account seasonal conditions and the status of the hive.
The natural product route
Organic beekeepers focus their attention of use of natural plant oils as putative miticides. Due to microbial action, these products, both natural oils – or their active constituents – and the organic acids, degrade very quickly in beehives, have a propensity to flavour honey and have encountered many problems in their being effectively applied: These topics are reviewed comprehensively in Smite the Mite Part III – The natural oils. By and large use of natural products is hampered by lack of regulatory approval, sometimes dubious efficacy and a lack of understanding as to how they might be most effectively applied. Sprigs of mint family plants, a few curls of cinnamon bark or a dribble of oil of wintergreen on a piece of blotting paper won’t work. There will be insufficient active constituent to kill mites or the applied material may never come into effective contact with mites.
Natural oils are also highly variable in composition and are dissipated within a few days so can ever only, well applied, kill free ranging mites. They are likely best employed to treat swarms and broodless bees say by spraying. We have visited thymol for which there is a large body of evidence demonstrating its miticidal efficacy and, in Smite the Mite Parts II and VI, we examined how its brood nest residence time can be greatly extended using impregnated wood fibre blocks.
The simple organic acid route
As effective alternatives to now widely failing synthetic arachnicides, the simple organic acids have emerged at the pointy end of mite treatment. There are a number of these acids (Table 2), but only a few have been evaluated for their effectiveness in controlling Varroa.
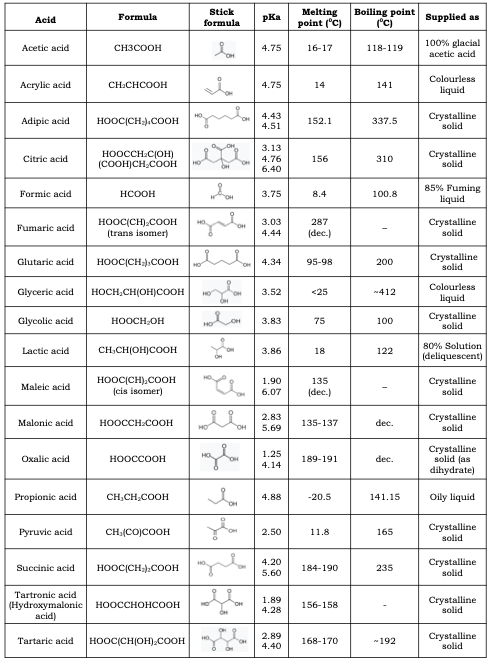
Table 2 Simple organic acids with notional mite target potential. While most have not been trialled several are of comparable acidity to oxalic and lactic acid and may persist longer in the hive.
Their common mode of action is solely contingent upon the presence of the acid (H3O+) hydronium ion (Oliver, R., 2017). However the acids vary widely in their acidity and physical properties, factors that either constrain their level of use or define their best mode of application. An emerging feature of natural product use – the acids are no exception – is the formulation of products that extend their active hive life. Both formic acid and oxalic acid have been deployed in such manner factors that have greatly improved their mite killing effectiveness.
Left field enter the common amino acids. All are amphoteric, that is they act as both acids and bases in water so neither behave as acids of bases though having a pKa1 ranging from 1.82 to 2.36. However for all intents and purposes they are fairly neutral as they absorb small amounts of added acid or alkali. Two of these basic building blocks are, like oxalic acid, both dicarboxylic acids and both are acidic: aspartic acid (pKa1 2.09) and glutamic acid (pKa1 2.19) (Figure 2). Monosodium glutamate (a simple salt of glutamic acid) of course is famed for its flavour enhancement quality in Asian cuisine. More surprisingly glutamic acid has been incorporated into a new generation arachnicide being developed by the United States Environmental Protection Agency (2025), one that mimics slow release oxalic acid in killing mites and that is on the agenda for approval.
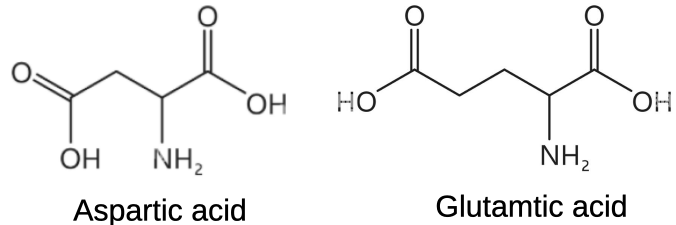
Figure 2 The dicarboxylic acid amino acids
Since organic acids have many commercial applications they are manufactured at scale and are generally available as the pure product or as a concentrated solution. Several acids have been registered for mite control, typically as gels, capsules, impregnated strips or as simple to use make up kits. None accumulate in bee products and several are present naturally in honey or in stored pollen. Lactic acid, by way of example, is employed by bees as a pollen preservative.
Let’s look at each of these acids and review their attributes to see how they can be best applied and under what conditions. Since mites are associated with brood or with nurse bees tending brood, treatments are best applied and are most effective when hives comprise brood boxes only. The alternative to chemical treatment, brood break manipulations are best implemented over summer (Uzunov et al., 2020, 2023) when any brood interruption will have minimal impact on normal colony function.
The volatile acids
Formic acid is emerging as an arachnicide of choice. It is effective against all honey bee mites but needs to be handled judiciously, both from an OH&S perspective and because improperly applied it will result in mass bee mortality.
Since formic acid and acetic acids are very volatile their evaporation rates need to be regulated, that is slowed down (Oliver, R., 2022c). Vinegar, containing 5-10 % acetic acid, has a sharp odour and the eye watering squashed bull ant provide warning enough that more concentrated versions of these acids must be handled with full PPE.
Siceanu and coworkers (2021) compared the efficacy of formic and acetic acids either vaporised into the brood nest (from 100 mL of formic acid solutions or glacial acetic acid absorbed onto cloth attached to walls and inner cover) or acids brushed onto mite laden capped brood (Table 3).
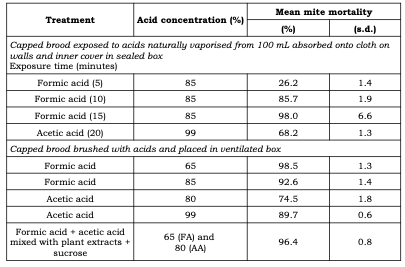
Table 3 Comparison of treatments of ten combs with capped brood to control Varroa employing formic and acetic acids. (Table adapted records published by Siceanu et al., 2021).
* Acid in open container on top of capped brood in sealed brood chamber.
These studies were conducted on ten frames of brood from which bees had been shaken so are only indicative of the effect of these acids on brood below wax cappings rather than on the nests of operating colonies. Critically the study defines the amounts of these acids needed to kill varroa effectively. Both the Siceanu team and van Engelsdorp and coworkers (2008) found that acetic acid or acetic acid in combination with formic acid were much less effective than formic acid alone.
An alternative approach to the use of gels to control formic acid release has been to regulate the flow of the acid from a reservoir. Such units wick acid onto a pad from which it evaporates over a time frame lasting up to about 20 days. The rate of acid delivery is somewhat less affected by temperature and relative humidity than from gels and is flow adjustable. Nevertheless their use is constrained by the same temperature envelope. Below about 9 0C formic acid is too slowly evaporated to kill mites. Above 30 0C bees are affected and in any case fan the acid away so mites are not effectively targeted.
Such is the Nassenheider evaporator (Rodney Beekeepers Club, 2025; Joachim Weiland Werkzeugbau GmbH & Co., 2014) (Figure 3), a simple device developed in Germany by beekeeper Bruno Becker in the 1990s (Oliver, 2007). It has been field tested in New Zealand (BeeQuip, 2025a) and is being trialled by Oliver (Wade, 2025) in California. BeeQuip (2025b) provides helpful instructions for the set up and installation of the evaporator.
The use of an evaporator to control Tropilaelaps would seem also to have great promise given that formic acid gel strips (Pettis et al., 2017) have been shown to be effective.

Figure 3 Nassenheider evaporator [Image https://agritura.com/en/products/evaporatore-nassenheider-professionale-1]
Many other formic acid release evaporators are available on the market for example as advertised in Denmark (Blive Biavler, 2025).
Cabbri and coworkers (2023) compared the performance of the Nassenheider evaporator with that of the in-frame based Aspro-Novar-Form evaporator. They showed that while their efficacy still varied with ambient conditions both performed comparably.
Satta and coworkers (2005) similarly compared the effectiveness of a formic acid gel (BeeVar) with wicked formic acid (Liebig-Dispenser [Andermatt Biovet GmbH, Lörrach, Germany], a graduated container inverted over a paper wick that could be trimmed to slow delivery. They achieved a 94-100% varroa mite kill. The gel released 5-9 g of formic acid per day while the wick evaporator (with a higher bee mortality) delivered 26-35 g of formic acid daily, reducable by wick trimming.
Steube et al. (2021) employed both Liebig dispensers and Nassenheider [Joachim Weiland Werkzeugbau GmbH & Co KG, Hoppegarten, Germany] evaporators in a long term study to evaluate the efficacy of 60 and 85 % formic acid employing both single and double brood chamber colonies. They concluded that using the more concentrated acid reduced the dependence on temperature for mite killing efficacy, but pointed to ambient conditions being the main factor contributing to quite variable mite kill rates.
Pietropaoli and Formato (2018, 2019) compared the efficacy of different methods of mite exposure to formic acid (evaporation of the acid from a polysaccharide gel, or regulated drip of 65% formic acid onto an evaporation pad). Their studies emphasised the problems associated with regulating formic acid release and the variability in mite kill rates depending of the mode of formic acid release, the use of gels versus the use of an evaporator. They emphasised the oft cited problem of queen loss that Oliver has attributed to the distressed reaction of worker bees to the acid where the bees not the chemical knocks the queen off.
Clearly much needs to be learnt about the use of formic acid as a miticide. Overall it would appear that using the acid under optimal temperature conditions, the low to mid twenties, and that regulating the acid delivery rate employing one of the better designed wick to evaporation pad units will become the default practice.
The crystalline acids
These acids, solids or solutions of deliquescent solids, are mobilised to target mites and their host bees so they come into contact with the acids. This delivery has been achieved in several ways: brushing solutions of the acids onto brood combs, syringing solutions of the acids into the gap between brood combs, strip formulations of acids (typically those admixed with glycerine) laid over brood combs or as sublimated acids that envelop the brood nest.
In perhaps the simplest of all treatment applications, a battery operated vaporiser, charged with a 2 g shot of oxalic acid is fogged directly into double brood box hive entrance with the push of a button. The treatment lasts for about 30 seconds and the entrance is towelled over briefly to allow the acid to settle. Oliver suggests that the acid will adhere better and be more effective if the vapour stream is warm.
The use of an oxalic acid evaporator is an art form so requires careful study to make it effective and safe to use. The most useful guide I’ve come across (BeeQuip, 2025c) provides instruction for multiple hive treatment, safety measures, maintenance of gear and getting the best out of rechargeable battery packs.
In more standard dribble application solutions of 3.4% oxalic or 15% lactic acid (Klepsch, 1984; Ibrahim and Ezzat, 1993; Kraus and Berg, 1994; Brødsgaard et al.,1997; Gregorc and Planinc, 2002; Gregorc and Poklukar 2003; Rademacher and Harz, 2006; Girisgin and Aydin, 2010; Kosch et al., 2025) are syringed between brood frames or sprayed onto brood comb faces. The treatment is best adapted to post harvest autumn conditions – when honey supers have been removed. It is also ideal for swarm treatment or to rid shook bees of mites.
Since introduced acids persist for a few days at most they only target phoretic mites. Milani (2001) evaluated the activity of oxalic and citric acids sprayed onto brood at various stages of development concluding that the highly acidic oxalic acid was more lethal and was enhanced with added sugar syrup. Vilarem and coworkers (2023) showed that a suite of acids including citric, tartaric, lactic and formic acids resulted in mite detachment from their host but did not ascertain their miticidal efficacy in vivo.
Extended oxalic acid formulations, those containing glycerine, are absorbed onto cellulose based cardboard or dishcloth (Branchiccela et al., 2025; Maggi et al., 2016, Oliver, 2022a, 2022b) or are formulated as gels. The prepared strips or gels are positioned over brood combs to maximise their contact with crawling nurse bees. This ensures bees are coated with sufficient acid to kill attached mites.
Which acid to use
Since the strength of acids varies considerably we must choose an acid fit for purpose, one that if appropriately applied will kill most mites but as few bee larvae or adult bees as possible. The pKa of an acid is a simple measure of this strength, just as pH is a simple measure of the actual amount of acid in solution – the lower the value the stronger the acid or the amount of acid present. It is therefore not surprising that amongst the simple and most readily available simple organic acids (see Table 1) that formic and oxalic acids are the acids of choice. Formic acid, perhaps also acetic acid, has one further conferred advantage. Formic acid is a tiny molecule that permeates brood cappings, so targets mites as they breed on pupae inside capped cells.
With the discovery of the capacity of formic acid to kill Varroa mites (Ritter et al., 1980) and the subsequent finding that treatment was ineffectual at low temperature but lethal to bees and ineffectual above 30 0C (Wachendörfer et al., 1985; Imdorf et al., 2003) came a rash of treatments employing gels (Mutinelli et al., 1994; Eguaras et al., 2001a, 2001b, 2003; Satta et al., 2005; Giusti et al., 2017; Avila-Ramos et al., 2010). The gels regulated the release of the acid increasing mite exposure time while avoiding excessive bee mortality.
The current gel formulations are Mite Away and the seemingly superior polysaccharide based FormicPro. FormicPro releases formic acid over a 20 day period, much of it in the first 10 days. The key and emerging alternative use of evaporators (discussed above) is achieving high mite kill rates and is a lower cost solution for mite control.
A mitey way forward
In the short to medium term Australian beekeepers will learn to accept what the global beekeepers have long experienced, loss of bees to Varroa. In the early stages of our mite journey it is inevitable that some of our naïve bees will fall victim to sudden influxes of mites. Those losses will most likely occur in apparently healthy colonies heading into winter. Counterintuitively we will also discover that Newcastle district beekeepers have been able to keep bees tolerating high varroa mite loads, a consequence of the absence of debilitating deformed wing virus.
Having to deal with other mites, Acarapis woodi (tracheal) mites (likely not a serious pest) and giant honey bee Tropilaelaps mercedesae mites will be another challenge, though focusing on treatments that will target all mites would, no doubt, help.
Ultimately we will all have to find our own way to control the mite blight. While many will choose to use synthetic miticides such as Bavarol, the problems of contaminating bee gear and wax combs, their reputation for becoming miticide resistant and the sheer cost of treatment, would recommend that alternative means be found for mite control. The North American and European trend to employ organic acids as cheap and effective miticides has considerable merit.
Readings
Anderson, D.L. and Roberts, J.M.K. (2013). Standard methods for Tropilaelaps mites research. Journal of Apicultural Research 52(4):1-16. https://doi:10.3896/ibra.1.52.4.21
Apimonda Tropilaelaps webinar. (22 April 2025). Expert talks on Tropilaelaps mites. https://youtu.be/K-yRRHLROdc
Avila-Ramos, F., Otero-Colina, G., Sánchez-Arroyo, H., Santillán-Galicia, M.T. and Tecante, A. (2010). A gel formulation of formic acid for control of Varroa destructor. In Trends in Acarology: Proceedings of the 12th International Congresspp.545-549. Springer Netherlands. https://www.researchgate.net/publication/273259562_A_gel_formulation_of_formic_acid_for_control_of_Varroa_destructor
BeeQuip (2025a). Field trial with the Nassenheider professional evaporator. https://beequip.nz/blogs/blog/field-trial-with-the-nassenheider-evaporator
BeeQuip (2025b). How to set up the Nassenheider evaporator. https://www.youtube.com/watch?v=L_1rCjODXk8
BeeQuip (2025c). InstantVap operation guide. https://beequip.nz/pages/instantvap-operation-guide
Blive Biavler (2025). New formic acid evaporators. https://www.biavl.dk/medlemmer/videnbank/beekeeping-in-denmark-2/varroa-in-english/new-formic-acid-vaporizers/
Branchiccela, B., Díaz-Cetti, S., Ramallo, G. and Mendoza, Y. (2025). Oxalic acid in cellulose strips: Towards an efficient and sustainable approach for the control of Varroa destructor. Apidologie 56(1):21. https://link.springer.com/journal/13592/volumes-and-issues/56-1
Brødsgaard, C.J., Hansen, H. and Hansen, C.W. (1997). Effect of lactic acid as the only control method of varroa mite populations during four successive years in honey bee colonies with a brood free period. Apiacta 32(3):81-88. https://www.fiitea.org/cgi-bin/index.cgi?sid=&zone=cms&action=search&categ_id=208&search_ordine=descriere
Cabbri, R., Danielli, S. and Galuppi, R. (2023). Treatment based on formic acid for Varroa destructor control with two different evaporators: Efficacy and tolerability comparison. Journal of Apicultural Research 62(5):999-1006. https://sci-hub.sidesgame.com/10.1080/00218839.2021.1920234
Cushman, D. (accessed 5 April 2025). The Nassenheider evaporator. http://www.dave-cushman.net/bee/nassenheider.html
Dietemann, V., Ellis, J.D. and Neumann, P. (2013a). The COLOSS Beebook Volume II, Standard methods for Apis mellifera pest and pathogen research: Introduction. Journal of Apicultural Research 52(4):1-4. https://doi:10.3896/ibra.1.52.4.16
Dietemann, V., Nazzi, F., Martin, S.J., Anderson, D.L., Locke, B., Delaplane, K.S., Wauquiez, Q., Tannahill, C., Frey, E., Ziegelmann, B., Rosenkranz, P. and Ellis, J.D. (2013b). Standard methods for Varroa research. Journal of Apicultural Research 52(1):1-54. https://www.wellesu.com/10.3896/ibra.1.52.1.09
Eguaras, M., Del Hoyo, M., Palacio, M.A., Ruffinengo, S. and Bedascarrasbure, E.L. (2001a). A new product with formic acid for Varroa jacobsoni Oud. control in Argentina. I. Efficacy. Journal of Veterinary Medicine, Series B 48(1):11-14. https://www.wellesu.com/10.1111/j.1439-0450.2001.00418.x
Eguaras, M.J., Labattaglia, M., Faverin, C., Del Hoyo, M., Palacio, M.A., Carrin, M., Rodriguez, G., Ruffinengo, S.R. and Bedascarrasbure, E. (2001b). Varroa jacobsoni control with formic acid used in different application ways in subtropical and temperate climates. https://ri.conicet.gov.ar/bitstream/handle/11336/41877/CONICET_Digital_Nro.ec6d6f7b-806a-465d-9365-ea9c77065691_B.pdf?sequence=5&isAllowed=y
Eguaras, M., Palacio, M.A., Faverin, C., Basualdo, M., Del Hoyo, M.L., Velis, G. and Bedascarrasbure, E. (2003). Efficacy of formic acid in gel for Varroa control in Apis mellifera L.: Importance of the dispenser position inside the hive. Veterinary Parasitology 111(2-3):241-245. https://www.wellesu.com/https://doi.org/10.1016/s0304-4017(02)00377-1
Gill, M.C., Chuttong, B., Davies, P., Etheridge, D., Panyaraksa, L., Tomkies, V., Tonge, G. and Budge, G.E. (2024). Assessment of the efficacy of field and laboratory methods for the detection of Tropilaelaps spp. PloS ONE 19(9):p.e0301880. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0301880
Gill, M. (May 2024). An underestimated threat? Bee Culture. https://beeculture.com/tropilaelaps-3/
Girisgin, A.O. and Aydin, L. (2010). Efficacies of formic, oxalic and lactic acids against Varroa destructor in naturally infested honeybee (Apis mellifera L.) colonies in Turkey. Kafkas Üniversitesi Veteriner Fakültesi Dergisi 16(6):941-945. https://vetdergikafkas.org/uploads/pdf/pdf_KVFD_813.pdf
Giusti, M., Sabelli, C., Di Donato, A., Lamberti, D., Paturzo, C.E., Polignano, V., Lazzari, R. and Felicioli, A. (2017). Efficacy and safety of Varterminator, a new formic acid medicine against the varroa mite. Journal of Apicultural Research 56(2):162-167. https://doi:10.1080/00218839.2017.1291207
Gregorc, A. and Planinc, I. (2002). The control of Varroa destructor using oxalic acid. The Veterinary Journal 163(3):306-310. https://doi:10.1053/tvjl.2001.0675
Gregorc, A. and Poklukar, J. (2003). Rotenone and oxalic acid as alternative acaricidal treatments for Varroa destructorin honeybee colonies. Veterinary Parasitology 111(4):351-360. https://sci-hub.sidesgame.com/10.1016/s0304-4017(02)00408-9
Ibrahim, S.H. and Ezzat, T.H. (1993). Zur wirkung von milchsäure auf den Varroa-befall der biene. [Studies on the effect of lactic acid on Varroa infestation of the bee.] Anzeiger fur Schadlingskunde, Pflanzenschutz, Umweltschutz 66(2):31-32. https://doi:10.1007/BF01909139
Imdorf, A., Charrière, J.D., Kilchenmann, V., Bogdanov, S. and Fluri, P. (2003). Alternative strategy in central Europe for the control of Varroa destructor in honey bee colonies. Apiacta 38(3):258-278. https://www.fiitea.org/foundation/files/2003/Imdorf%202.pdf
Jenne, B. (accessed 28 April 2025). Latest greatest mite killing machine: Instant Vap compact. https://www.youtube.com/watch?v=NoHryQu2tGg&t=613s
Joachim Weiland Werkzeugbau GmbH & Co. KG (2014). Nassenheider Verdunster Professional, Langzeitverdunster für Ameisensäure zur sicheren Behandlung der Varroose. https://archive.org/details/gebrauchsanweisung-nv-professional-2015/mode/2up
Klepsch, A. (1984). Treatments of Varroa disease by spraying colonies with lactic-acid. Apidologie 15(3):261-262. [In German with an English summary] In Working group of the apicultural institutes in Western Germany: Report on the meeting at Stuttgart-Hohenheim, 13.-15.3.1984. https://www.apidologie.org/articles/apido/pdf/1984/03/Apidologie_0044-8435_1984_15_3_ART0001.pdf
Kosch, Y., Mülling, C. and Emmerich, I.U. (2025). Assessment of resistance of Varroa destructor to formic and lactic acid treatment: A systematic review. Veterinary Sciences 12(2):144. https://www.researchgate.net/publication/388876946_Assessment_of_Resistance_of_Varroa_destructor_to_Formic_and_Lactic_Acid_Treatment-A_Systematic_Review
Kraus, B. and Berg, S. (1994). Effect of a lactic acid treatment during winter in temperate climate upon Varroa jacobsoni Oud. and the bee (Apis mellifera L.) colony. Experimental and Applied Acarology 18:459-468. https://www.academia.edu/58781030/Effect_of_a_lactic_acid_treatment_during_winter_in_temperate_climate_upon_Varroa_jacobsoni_Oud_and_the_bee_Apis_mellifera_L_colony
Maggi, M., Tourn, E., Negri, P., Szawarski, N., Marconi, A., Gallez, L., Medici, S., Ruffinengo, S., Brasesco, C., De Feudis, L. and Quintana, S. (2016). A new formulation of oxalic acid for Varroa destructor control applied in Apis mellifera colonies in the presence of brood. Apidologie 47(4):596-605. https://sci-hub.sidesgame.com/10.1007/s13592-015-0405-7
Milani, N. (2001). Activity of oxalic and citric acids on the mite Varroa destructor in laboratory assays. Apidologie 32(2):127-138. https://www.apidologie.org/articles/apido/pdf/2001/02/milani.pdf
Mutinelli, F., Cremasco, S. and Irsara, A. (1994). Formic acid in the control of varroatosis: A practical approach. Journal of Veterinary Medicine Series B 41(1-10):433-440. https://doi.org/10.1111/j.1439-0450.1994.tb00248.x
Oliver, R. (2007). The Arsenal: Natural treatments Part 1. Scientific Beekeeping. https://scientificbeekeeping.com/the-arsenal-natural-treatments-part-1/
Oliver, R. (2017). Extended-release oxalic acid progress report #2. Scientific Beekeeping. https://scientificbeekeeping.com/extended-release-oxalic-acid-progress-report-2/
Oliver, R. (2022a). Extended-release oxalic (OAE) udate Part 1. Scientific Beekeeping. https://scientificbeekeeping.com/7701-2/
Oliver, R. (2022b). Extended-release oxalic (OAE) udate Part 2. Scientific Beekeeping. https://scientificbeekeeping.com/2022-extended-release-oxalic-oae-update-part-2/
Oliver, R. (2022c). Formic Pro in hot weather: Slowing the rate of vapor release. Scientific Beekeeping. https://scientificbeekeeping.com/formic-pro-in-hot-weather-slowing-the-rate-of-vapor-release/
Oliver, R. (2023). Oxalic acid treatment table. Scientific Beekeeping. https://scientificbeekeeping.com/oxalic-acid-treatment-table/
Pettis, J.S., Rose, R., Lichtenberg, E.M., Chantawannakul, P., Buawangpong, N., Somana, W., Sukumalanand, P. and van Engelsdorp, D. (2013). A rapid survey technique for Tropilaelaps mite (Mesostigmata: Laelapidae) detection. Journal of Economic Entomology 106(4):1535-1544. https://academic.oup.com/jee/article/106/4/1535/2962118
Pettis, J.S., Rose, R. and Chaimanee, V. (2017). Chemical and cultural control of Tropilaelaps mercedesae mites in honeybee (Apis mellifera) colonies in Northern Thailand. PloS ONE 12(11):p.e0188063. https://journals.plos.org/plosone/article/file?id=10.1371/journal.pone.0188063&type=printable
Pietropaoli, M. and Formato, G. (2018). Liquid formic acid 60% to control varroa mites Varroa destructor in honey bee colonies Apis mellifera: Protocol evaluation. Journal of Apicultural Research 57(2):300-307. https://doi:10.1080/00218839.2017.1376767
Pietropaoli, M. and Formato, G. (2019). Acaricide efficacy and honey bee toxicity of three new formic acid-based products to control Varroa destructor. Journal of Apicultural Research 58(5):824-830. https://www.tandfonline.com/doi/pdf/10.1080/00218839.2019.1656788
Rademacher E. and Harz M. (2006). Oxalic acid for the control of varroosis in honey bee colonies–a review. Apidologie37(1):98-120. https://www.apidologie.org/articles/apido/pdf/2006/01/M6010.pdf
Ramsey, S. (2018). Infestation of honey bees with Tropilaelaps spp. Chapter 2.2.6 OIE Terrestrial Manual. https://www.woah.org/fileadmin/Home/eng/Health_standards/tahm/2.02.06_TROPILAELAPS.pdf
Ritter, W., Ruttner, F., Maul, V., Petersen, N., Wissen, W., Koeniger, N. and Rau, C. (1980). Die Varroatose der Honigbiene Neue Wege in der Behandlung der Varroatose: 1. Ameisensaeure. Allgemeine Deutsche Imkerzeitung 14(5):151-155. https://pascal-francis.inist.fr/vibad/index.php?action=getRecordDetail&idt=PASCALZOOLINEINRA8110602362
Rodney Beekeepers Club (accessed 5 April 2025). Nassenheider evaporator professional: Longterm-evaporator for formic acid for the safe treatment of varroasis. https://rodneybeekeepersclub.co.nz/wp-content/uploads/2015/08/manual-nassenheider.pdf
Satta, A., Floris, I., Eguaras, M., Cabras, P., Garau, V.L. and Melis, M. (2005). Formic acid-based treatments for control of Varroa destructor in a Mediterranean area. Journal of Economic Entomology 98(2):267-273. https://www.wellesu.com/10.1603/0022-0493-98.2.267
Siceanu, A., Cǎuiǎ, E., Vişan, G.O. and Cǎuiǎ, D. (2021). The sustainable control of varroosis (Varroa destructor) by treatment of capped honeybee brood using organic volatile acids and innovative procedures. Scientific Papers. Series D. Animal Science 64(1):382-399. https://animalsciencejournal.usamv.ro/pdf/2021/issue_1/Art55.pdf
Steube, X., Beinert, P. and Kirchner, W.H. (2021). Efficacy and temperature dependence of 60% and 85% formic acid treatment against Varroa destructor. Apidologie 52(3):720-729. https://link.springer.com/content/pdf/10.1007/s13592-021-00859-5.pdf
United States Environmental Protection Agency (29 May 2025). EPA proposes to register new pesticide for Varroa mite control. https://www.epa.gov/pesticides/epa-proposes-register-new-pesticide-varroa-mite-control
Uzunov, A., Kovačić, M., Prešern, J., Pietropaoli, M., Hatjina, F., Pavlov, B., Charistos, L., Formato, G., Galarza, E. and Gerula, D. (2020). Summer brood interruption as integrated management strategy for effective Varroa control in Europe. Journal of Apicultural Research 59(5):764-773. https://sci-hub.sidesgame.com/10.1080/00218839.2020.1793278
Uzunov, A., Gabel, M. and Büchler, R. (2023). Summer brood interruption for vital honey bee colonies: Towards sustainable Varroa control using biotechnical methods. Apoidea Press.
Uzunov, A., Gabel, M. and Büchler, R. (2025a). Summer brood interruption: Why and how. The Beekeepers Quarterly 159:17-19.
Uzunov, A., Janashia, I., Chen, C., Costa, C. and Kovačić, M. (2025b). A scientific note on rapid brood decapping: A method for assessment of honey bee (Apis mellifera) brood infestation with Tropilaelaps mercedesae. Apidologie 56(2):40. BioRΧiv https://www.biorxiv.org/content/10.1101/2024.10.09.616962v1.full
Uzunov, A., Janashia, I., Chen, C., Costa, C. and Kovačić, M. (2025c). Rapid brood decapping for Tropilaelaps mercedesae detection. Bee World 1-6. https://www.tandfonline.com/doi/full/10.1080/0005772X.2025.2483730?src=
van Engelsdorp, D., Underwood, R.M. and Cox-Foster, D.L. (2008). Short-term fumigation of honey bee (Hymenoptera: Apidae) colonies with formic and acetic acids for the control of Varroa destructor (Acari: Varroidae). Journal of Economic Entomology 101(2):256-264. https://www.wellesu.com/10.1603/0022-0493(2008)101%5B256:sfohbh%5D2.0.co;2
Veet® Australia (accessed 6 May 2025). Cold wax strips. https://www.veet.com.au/products/cold-wax-strips/
Vilarem, C., Piou, V., Blanchard, S., Vogelweith, F. and Vétillard, A. (2023). Lose your grip: Challenging Varroa destructor host attachment with tartaric, lactic, formic, and citric acids. Applied Sciences 13(16):p.9085. https://www.mdpi.com/2076-3417/13/16/9085
Wachendörfer, G., Fijalkowski, J., Kaiser, E., Seinsche, D. and Siebentritt, J. (1985). Labor-und Feldversuche mit der Illertisser Milbenplatte als neue Anwendungsform der Ameisensäure im Rahmen der Varroatose-Bekämpfung. Apidologie 16(3):291-306. https://www.apidologie.org/articles/apido/pdf/1985/03/Apidologie_0044-8435_1985_16_3_ART0005.pdf
Wade, A. (March-April 2025). Personal correspondence with Randy Oliver.
