Image: Travel online (2025)
Alan Wade
Keith Garvey (1981) tells us how his Uncle Harry made short work of a giant Australian bush tick:
I hurls the rum bottle and hits it [the tick] in the left eye, and slows it down enough for me to scramble up on the wagon. The only thing I can get me hands on is a packet of Cooper’s Dip. I throws it at the tick, and it busts and the yellow powder showers all over him!
That did it! He starts to wobble and shake, then like a drunken man he staggers a few steps and collapses right on the camp fire. The last thing I remember before I passed out from shock was the blue flame flyin’ out of him as his natural gases exploded.
ttTicks and parasitic honey bee mites belong to the Acari arm of the arachnids. In this they are set apart from common a garden, 8-legged spiders, scorpions, solifuges (camel spiders) and oliopolones (daddy longlegs). Might we not use Cooper’s arsenic dip to kill Varroa and other troublesome bee parasites? Well, yes we could. The trouble is that it would also spell the death knell of our bees. What we really want are formulations that will kill mites, not take the bees with them.
But to make sense of any control program, we need to understand that a wide array of mites, many beneficial, those that have been found inhabiting honey bee nests. From there we can come back to the parasitic mites that have so devastated honey bees almost everywhere.
All those mites
raMercedes Delfinado (1963) was the first to recognise that the triumvirate of Acarapis, Varroa and Tropilaelaps would decimate western honey bees (Apis mellifera) and, in some cases, also farmed sub-species of the Asian honey bees (Apis cerana). In later describing the behaviour of mites associated with honey bees, Delfinado-Baker, Rath, and Boecking (1992) cite Eric Binns’ shrewd observation:
‘Why grow wings, said the mite, when you can thumb a ride on a passing fly [bee]? Some mites take up this specialised form of parasitism to reach an assured food supply, while others hitchhike to escape from a dying habitat.’
Eric S. Binns, 1976
Binns (1982) better known for his critical appraisal of phoresy, hitchhiking behaviour, notes that:
In bee-mite relationships in the honey bee Apis mellifera L. in Germany relatively few of 18 phoretic species had any ‘permanent’ relationship to the individual or the colony.
Michael Bush (2025) lists some 751 species of mites that have been found in honey bee colonies. Of these relatively few are phoretic and only a couple of handfuls are parasitic. Indeed most of the common mites are saprophytic (scavenging opportunists), unpaid house cleaners that remove fungal debris from the floor of tree hollow nests and the bottom boards of hives. You are most likely to find these mites in hive debris and not realise their important function in maintaining colony hygiene. Commonly found mites include Trichouropoda (Rubink et al.,1991; Delfinado-Baker et al.,1992), Pseudacarapis (Ochoa et al., 2003), Vairimorpha ceranae (Namin et al., 2024), and the widespread pollen mite Melittiphis alvearius (Barreto et al., 2004; Delfinado-Baker, 1994; Brandorf et al., 2024).
The parasitic mites
The parasitic mites that take a toll on honey bees are limited to a handful of genera: Acarapis, Euvarroa, Tropilaelaps and Varroa as well as the less well known Leptus from South America (Southcott, 1989; Wilson et al.,1987; Wilson et al., 1990; Martin and Correia-Oliveira, 2016) (Table 1).
| Mite order | Mite family | Parasite species | Host species |
| Astigmatina | Tarsonemidae | Acarapis woodi | Apis mellifera |
| Acarapis externus Acarapis dorsalis | Not known but found on Apis dorsata, Apis cerana and Apis mellifera in Asia | ||
| Mesostigmata | Varroidae | Varroa destructor | Apis cerana |
| Varroa jacobsoni Varroa rindereri Varroa underwoodi | Likely Apis cerana Apis koschevnikovi Apis cerana | ||
| Euvarroa wongsirii Euvarroa sinhai | Apis andreniformis Apis florea | ||
| Laelapidae | Tropilaelaps clareae Tropilaelaps mercedesae Tropilaelaps koenigerum Tropilaelaps thaii | Apis breviligula and Apis dorsata binghami Apis dorsata and Apis laboriosa Apis dorsata Apis laboriosa | |
| Prostigmata | Erythraeidae | Leptus ariel | Apis mellifera scuttelata |
Table 1 Phoretic honey bee (Apis mellifera) parasites.
Poona Chandra and coworkers (2021) describe the association of the phoretic mite Neocypholaelaps indica and other mites with Apis cerana. Whatever, controlling parasitic mites probably has the unintended effect of killing the full spectrum of hive mites.
For now the task at hand is Varroa control, one that is in sympathy with good beekeeping practice. Two other damaging mites, Varroa jacobsoni and Tropilaelaps mercedesae are but a short holiday flight or cruise ship trip away from our shores. Despite these mites initially crashing colonies in Papua New Guinea, few beekeeping magazines and news reports afford these ‘extraterrestrials’ any attention.
Amongst all honey bee pests, Tropilaelaps (tropi) mites – along with their viruses – are widely cited as the most destructive. Tropi mites outstrip Varroa in their capacity to kill honey bees by a country mile, largely as a result of their explosive breeding potential and their sheltered existence under capped brood. They have moved from the giant honey bees to Apis mellifera as well as to widely farmed Apis cerana honey bees and the dwarf honey bees. Their arrival anywhere and everywhere might be likened to a rat-driven black plague.
Tropi makes Varroa look gentle!
Randy Oliver (2025).
Recent reports on the advance of Tropilaelaps into Europe (Brandorf et al., 2024; Carreck, 2023, 2024; Janashia et al., 2024; Apimonda, 2025) were foreshadowed by Chantawannakul et al. (2018). Poor quarantine practices in eastern Europe signal that this mite will soon enough become endemic across the European western block. The Middle East, the Americas, the unaffected parts of the Pacific, including Australia and New Zealand, are next in line.
In tropical climes Tropilaelaps normally displaces Varroa. In colder regions, where there is a well-defined brood break, Tropilaelaps does not replicate or survive winter so its populations crash. While apiaries may become seemingly tropi free, a few mites always survive and return to breed up quickly as soon as brood reappears.
So what happens when varroa and tropilaelaps mites are present and become endemic and infect the same hive? The populations of both seesaw widely so colonies under stress are prone to collapse year round. At the commencement of a normal season Varroa mite populations grow quickly and continue to develop through to late autumn when the bee colony naturally down regulates. Mite numbers remain high in autumn so the infestation rate, mites per bee or per pupa, actually increases. In this scenario winter losses of colonies, bees heavily parasitised by Varroamites and weakened by viruses, can be extreme. Colony losses in the United States of America were typically as high as 60% in calendar 2024.
Tropilaelaps, when also present, behaves differently. At the start of the season tropi mite numbers are vanishingly low. However since tropilaelaps can reestablish itself very quickly it can outcompete Varroa – a double jeopardy scenario. In any situation where mite control measures are less than optimal the problem morphs into year round colony demise.
The Asia-Pacific multi-mite scenario
The local scene
In a recent varroa field day held in Canberra, Torsten Engelhardt (2025) made a few pertinent observations. Firstly he noted that not all the reported Sydney hive collapses were solely attributable to Varroa. Small Hive Beetle (SHB) has formed an unholy alliance with Varroa and colonies are dying like flies with SHB slime outs. This condition is replicated in tropical Hawaii (Oliver, March 2025): Varroa weakens hives but it is the beetle that finish them off. Other hives, under mite pressure, are also absconding leaving full boxes of brood and stores behind. Oliver records that this happens routinely in North America though, with deformed wing virus, the brood left behind is invariably in poor condition.
What do these sudden losses to Varroa tell us? Firstly, as well as instituting regular mite checks, installing extra beetle traps – as I have done – would appear to be worthwhile. In my experience there is nothing much worse than the prospect of cleaning up slimed out brood. I am also requeening with chalkbrood hygienic stock. Recognising that Varroa will do most of the damage, I am inclined to the view that healthy bees with good winter stores will give my bees a better than even chance when, next spring, we will all be dealing with the mites.
The near Asian-Pacific scene
Some beekeepers will be surprised to discover that Australia, Papua New Guinea, the Solomon Islands, Fiji and most of the Pacific remain free of deformed wing virus (Roberts, 2017). Better known is that Varroa jacobsoni is widespread and that New Zealand and Tonga are blighted with Varroa destructor and deformed wing virus (Figure 1).
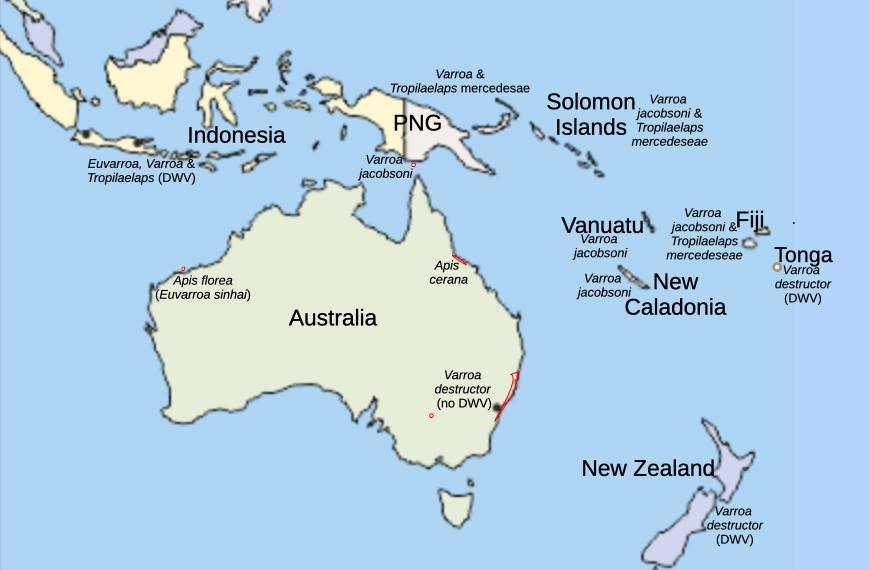
Figure 1 Present near Pacific honey bee-mite status.
Note: Only New Zealand and Tonga host Varroa with DWV (Roberts et al., 2020b).
What does our near neighbour mite situation portend? Tropilaelaps (the tropi mite) is as resilient as is any bush tick and its arrival is described by USDA’s Samuel Ramsey (2024) as:
‘…a fate worse than Varroa’.
American honey bee researcher Randy Oliver (2024) concurs:
‘…if we get Tropilaelaps it is is going to make Varroa look like nothing. It is an absolutely devastating parasitic mite far worse of a problem than the Varroa mite.’
Tropilaelaps mercedesae moved across from Apis dorsata and Apis laboriosa to Apis mellifera colonies wherever hives were colocated and as long as 100 years ago. The mite has recently spread northwards (Brandorf et al., 2024; Janashia et al., 2024) through China and into Russia. Namin and coworkers (2024) have tracked its genetic variations and phylogenetic patterns across its extended northern range.
The same species of Tropilaelaps has also moved eastward from the Indonesian Archipelago to Papua New Guinea, the Solomon Islands and Fiji and poses an existential risk to bees across the Pacific including Australia and New Zealand.
The damage to Australian beekeeping is currently limited to Varroa destructor in New South Wales. It has recently spread to Victoria and Queensland and it seems inevitable that Australia and New Zealand will suffer not only from the Varroa destructor mite but also import three other mites, Varroa jacobsoni, Tropilaelaps mercedesae and Tropilaelaps clareae (Table 1) though, to date, T. clareae has only parasitised Apis mellifera where it is sympatric with original host giant honey bees.
| Destructive honey bee mites | Original hosts | Other hosts |
| Tropilaelaps clareae | Apis breviligula and Apis dorsata binghami | Apis mellifera and Apis cerana in the Phillipines and the Sulawesi |
| Tropilaelaps mercedesae | Apis dorsata and Apis laboriosa | Apis mellifera, Apis cerana and Apis florea |
| Varroa destructor | Apis cerana | Korean and Japanese halotypes only on Apis mellifera |
| Varroa jacobsoni | Apis cerana japonica | Apis mellifera and Apis dorsata |
Table 1 Destructive honey bee mites.
The Tropilaelaps mites
Of all the mites Tropilaelaps mercedesae is of the greatest concern. It was originally confused with Tropilaelaps clareae but soon reclassified as a separate species (Anderson and Morgan, 2007). Widespread on the southern Asian continent and in Indonesia, and hosted by Apis dorsata and Apis laboriosa, Tropilaelaps mercedesae is spreading globally (Figure 2, Figure 3c). The record ofTropilaelaps clareae being in Burma and Pakistan (Delfinado-Baker, 1982) is incorrect and is an early record of Tropilaelaps mercedesae.
Tropilaelaps clareae, the type species, was first identified from Apis mellifera colonies and local field rats in the Philippines (Delfinado and Baker, 1961) and later found as a separate serotype in the Sulawesi. It is now widespread in Indonesia (Figure 3a). It too is a very serious pest of Apis mellifera. Importantly however, Tropilaelaps clareae, though parasitising Apis mellifera, has only done so where it is sympatric with its native giant honey bee hosts.
Tropilaelaps thaii (Figure 3d) hosted and restricted to Apis laboriosa has only been found in Vietnam (Anderson and Morgan, 2007) but these researchers suggest it is likely to be more widespread.
A related species Tropilaelaps koenigerum was first found in Sri Lanka (Delfinado-Baker and Baker, 1982; Koeniger, et al., 1983) but is also now recognised as being more widespread (Figure 3b). Neither Tropilaelaps thaii nor Tropilaelaps koenigerum have have crossed over to Apis mellifera or Apis cerana.
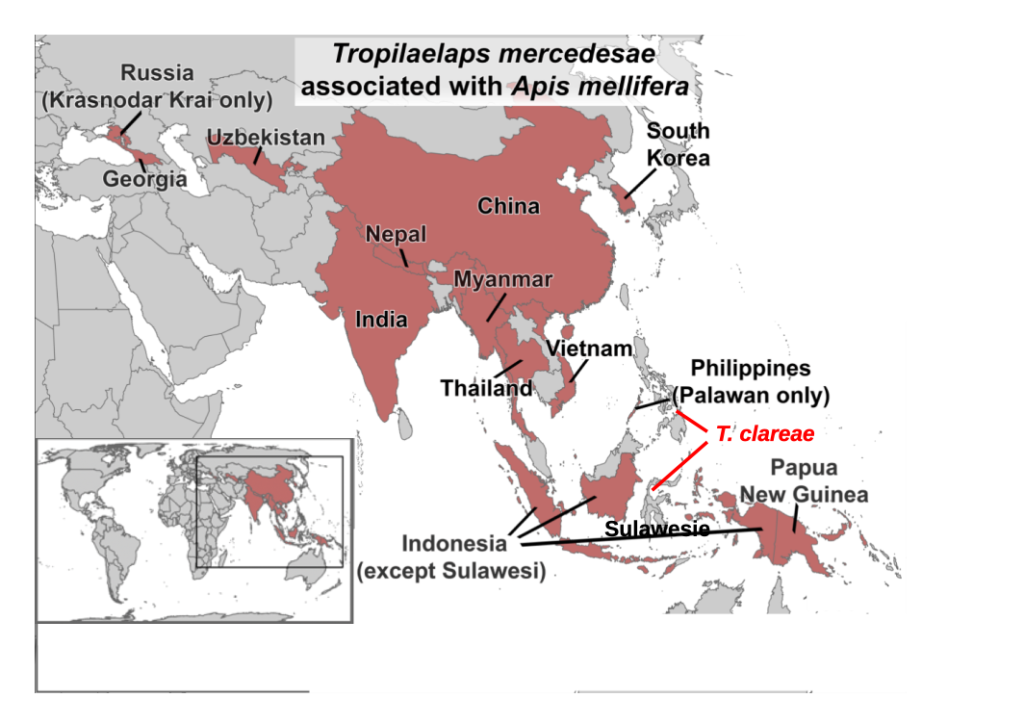
Figure 2 Global distribution of Tropilaelaps mercedesae. Redrawn after Jordan and Rogers (2024), showing also where also damaging Tropilaelaps clareae was first discovered. Similar maps for distribution of Tropilaelaps mercedesae abound (Direct Science, 2025; The National Bee Unit,2017; Melissocosmos, 2025; Gill, 2025).
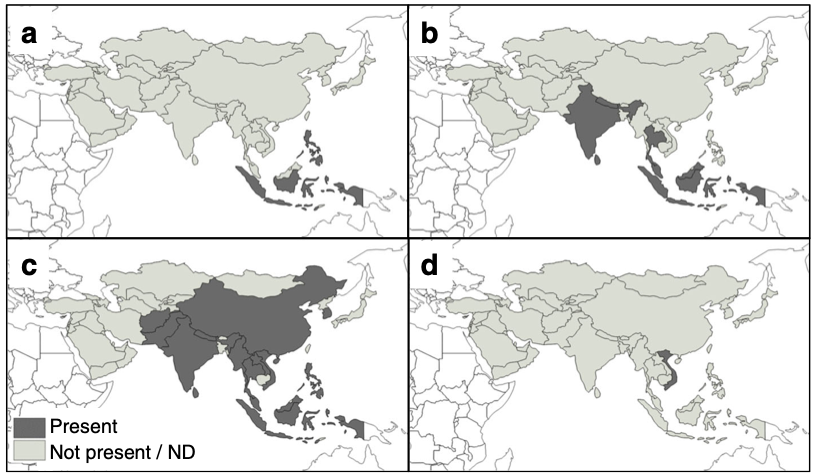
Figure 3 Contemporary distribution of Tropilaelaps mites: a Tropilaelaps clareae; b Tropilaelaps koenigerum; c Tropilaelaps mercedesae; and d Tropilaelaps thaii.
Source: Chantawannakul et al. (2016).
Chantawannakul and coworkers (2016) describe the dynamic relationship between the Varroa and Tropilaelaps affecting Apis mellifera colonies living sympatrically with Apis cerana and with the original host Apis dorsata group:
Tropilaelaps mercedesae is more prevalent in many Asian Apis mellifera colonies than Varroa destructor; and conversely
Varroa destructor is more competitive than Tropilaelaps mercedesae in temperate climates.
Natural mite host defences
Oldroyd and Wongsiri (2009) describe the natural defence mechanisms that Asian honey bees have mounted to control their Varroa, Euvarroa and Tropilaelaps mite populations. The propensity of most Asian bees to swarm regularly, in many instances to abscond, and in some cases to seasonally migrate to follow flowering plants, break brood rearing. All are undoubtably major factors in their achieving mite control. As significantly both the giant honey bees and the cavity dwelling Asian honey bee native hosts have very effective auto (self) and allo (communal) grooming behaviours (Büchler et al.,1992; Delfinado-Baker et al.,1992), limit all mite development to drone brood and have well developed traits for detection and removal (or imprisonment) of parasitised larvae. Such traits are expressed more weakly in European honey bees though Africanised honey bees (Johnson, 2023; Calderón et al., 2010; Carneiro et al., 2007) and African honey bee races of Apis mellifera have quickly adapted to varroa mites and may well develop tolerance of tropilaelaps mites. While the mechanisms for resistance are poorly understood, African races of bees have effective grooming and hygienic behaviour and mite fertility is intrinsically low.
So what other lessons might we learn about Tropilaelaps control from their natural giant bee hosts? Firstly we have a preemptory warning from the UK Bee Improvement and Bee Breeders Association (Gill, 2025):
In areas where both Tropilaelaps and Varroa are present it has been reported that Tropilaelaps are a far more damaging pest than Varroa and cause colony losses of between 50% to 80%. This is in part due to their lifecycle and more rapid reproductive rate. Tropilaelaps can mate outside of brood cells and unmated females can reproduce via deuterotokous parthenogenesis (de Guzman et al., 2018), a form of asexual reproduction whereby unfertilised eggs can develop into either males or females.
Elsewhere de Guzman and coworkers (2017) put the threat of Tropilaelaps into even clearer perspective:
For much of the world, Varroa destructor single-handedly inflicts unsurmountable problems to A. mellifera beekeeping. However, A. mellifera in Asia is also faced with another genus of destructive parasitic mite, Tropilaelaps. The life history of these two parasitic mites is very similar, and both have the same food requirements (i.e., hemolymph [more correctly fat bodies] of developing brood. Hence, parasitism by Tropilaelaps spp., especially Tropilaelaps mercedesae and Tropilaelaps clareae, also results in death of immature brood or wing deformities in infested adult bees.
Despite the dependence of Tropilaelaps on the presence of brood for reproduction it can also reproduce on the brood of other honey bees other than Apis mellifera and the giant honey bees including Apis florea (Gill, 2025) and Apis cerana further facilitating its spread.
Overall the dynamic is one of either or both Varroa and Tropilaelaps inflicting major damage. With Varroa mite populations continue to develop even as colonies naturally down regulate their number in autumn. This results in winter loss of colonies exacerbated by bees weakened by mites, the classic colony collapse disorder. Tropilaelaps behaves differently. Its mite numbers quickly grow from vanishingly low to outcompete Varroa by mid season. This double whammy can morph into year round colony demise.
Tropi control
If we look at the Tropilaelaps mite very closely we find that it has a biological profile that makes it more damaging to bees than Varroa:
- its replication rate is almost double that of Varroa;
- its life system is almost entirely confined to living under protective brood cell cappings unlike Varroa where some 20% of mites are phoretic;
- it does not parasitise adult bees, can live as long as 50 days and may parasitise a number of larvae; and
- its mating and reproduction is not confined to the brood nest.
Lilian de Guzman and coworkers (2017) have shown that this mite can mate beyond the confines of the brood cell and it has a capacity to reproduce parthenogenically. While colonies or whole apiaries may become mite free in the absence of brood, a few mites nevertheless survive. The mechanism for survival is unknown but is hypothesised to be facilitated by diapause, feral or other hives maintaining low levels of brood rearing or survival on alternative hosts.
In a common scenario, control of both Varroa and Tropilaelaps will be necessary. de Guzman, Williams, Khongphinitbunjong and Chantawannakul (2017) outline how this might be done. They surveyed many studies (Mahmood et al., 2012; Raffique et al., 2012; Hoppe et al., 1989) of Tropilaelaps control spanning the efficacy and limitations of use of synthetic miticides, thymol, the organic acids extended to include various forms of brood cycle intervention. Other studies by this group (Chantawannakul et al., 2016; Khongphinitbunjong et al., 2015; Chantawannakul et al., 2018; de Guzman et al., 2018) focus further on the biology and control of Tropilaelaps alone. Khongphinitbunjong et al. (2015) point particularly to the loss of condition and deformities that Tropilaelaps inflicts of bees.
Roberts and coworkers (2019, 2020a, 2020b) report on low cost measures, mainly inducing brood breaks, to control Varroa and Tropilaelaps in Papua New Guinea and in the Solomons. In a similar finding Pettis and coworkers (2017) demonstrated that the simple act of either splitting or removing a nucleus from colonies reduced the overall mite load in both Varroa and Tropilaelaps infested colonies. Roberts et al. (2020b) have also observed that, in the absence of deformed wing virus, bees have adapted to Varroa jacobsoni – perhaps less destructive than Varroa destructor – and Tropilaelaps mercedesae to the extent that treatment free beekeeping is now possible. Uzunov and coworkers (2023; 2025) have developed elaborate plans of brood interruption to control varroa, techniques that should find wide application in controlling tropilaelaps mites.
Many slow release formulations of formic acid are effective in targeting the full spectrum of honey bee parasitic mites. In a defining trial Kochansky and Shimanuki (1999) employed a formic acid infused polyacrylamide gel to facilitate the release of the acid over a period of 2-3 weeks. It proved to be effective against Tropilaelaps, Varroa and the tracheal mite Acarapis. Hoppe and coworkers (1989) employed cardboard strips containing 20 g of formic acid at four day intervals to achieve a high kill rates of Varroa, Tropilaelaps and again Acarapis mites, a scheme that avoided significant damage to bee brood and adult bees including queens.
Readings
Anderson, D.L. and Morgan, M.J. (2007). Genetic and morphological variation of bee-parasitic Tropilaelaps mites (Acari: Laelapidae): New and re-defined species. Experimental and Applied Acarology 43(1):1-24. https://doi:10.1007/s10493-007-9103-0
Apimonda Tropilaelaps webinar. (22 April 2025). Expert talks on Tropilaelaps mites. https://youtu.be/K-yRRHLROdc
Binns, E.S. (1982). Phoresy as migration: Some functional aspects of phoresy in mites. Biological Reviews 57(4):571-620. https://doi.org/10.1111/j.1469-185X.1982.tb00374.x
Barreto, M., Burbano, M.E. and Barreto, P. (2004). The bee mite Melittiphis alvearius (Berlese)(Acari: Laelapidae) in Colombia, South America. Neotropical Entomology 33(1):107-108. https://www.researchgate.net/publication/250030081_The_bee_mite_Melittiphis_alvearius_Berlese_Acari_Laelapidae_in_Colombia_South_America
Brandorf, A., Ivoilova, M.M., Yañez, O., Neumann, P. and Soroker, V. (2024). First report of established mite populations, Tropilaelaps mercedesae, in Europe. Journal of Apicultural Research68(2):1-3. https://www.wellesu.com/10.1080/00218839.1963.11100070
Büchler, R., Drescher, W. and Tornier, I. (1992). Grooming behaviour of Apis cerana, Apis melliferaand Apis dorsata and its effect on the parasitic mites Varroa jacobsoni and Tropilaelaps clareae.Experimental and Applied Acarology 16(4):313-319. https://scispace.com/pdf/grooming-behaviour-of-apis-cerana-apis-mellifera-and-apis-4tddv4ts3o.pdf
Bush, M. (accessed 6 February 2025). Bee mites. https://bushfarms.com/beesmites.htm
Calderón, R.A., van Veen, J.W., Sommeijer, M.J. and Sanchez, L.A. (2010). Reproductive biology of Varroa destructor in Africanized honey bees (Apis mellifera). Experimental and Applied Acarology50(4):281-297. https://sci-hub.sidesgame.com/10.1007/s10493-009-9325-4
Carneiro, F.E., Torres, R.R., Strapazzon, R., Ramírez, S.A., Guerra Jr, J.C., Koling, D.F. and Moretto, G. (2007). Changes in the reproductive ability of the mite Varroa destructor (Anderson and Trueman) in Africanized honey bees (Apis mellifera L.) (Hymenoptera: Apidae) colonies in southern Brazil. Neotropical Entomology 36(6):949-952. https://www.scielo.br/j/ne/a/hZQsr7tqBsCs75DV4sWKzFQ/
Carreck, N.L. (2023). Is Tropilaelaps on the move? The Beekeepers Quarterly 152:8-9.
Carreck, N.L. (2024). Tropilaelaps in Russia and beyond. The Beekeepers Quarterly 157:22-23.
Chantawannakul, P., de Guzman, L.I., Li, J. and Williams, G.R. (2016). Parasites, pathogens, and pests of honeybees in Asia. Apidologie 47(3):301-324. https://doi.org/10.1007/s13592-015-0407-5
Chantawannakul, P., Ramsey, S., Khongphinitbunjong, K. and Phokasem, P. (2018). Tropilaelapsmite: An emerging threat to European honey bee. Current Opinion in Insect Science 26:69-75. https://doi.org/10.1016/j.cois.2018.01.012
de Guzman, L.I., Williams, G.R., Khongphinitbunjong, K. and Chantawannakul, P. (2017). Ecology, life history, and management of Tropilaelaps mites. Journal of Economic Entomology 110(2):319-332. https://www.wellesu.com/10.1093/jee/tow304
de Guzman, L.I., Phokasem, P., Khongphinitbunjong, K., Frake, A.M. and Chantawannakul, P. (2018). Successful reproduction of unmated Tropilaelaps mercedesae and its implication on mite population growth in Apis mellifera colonies. Journal of Invertebrate Pathology 153:35-37. https://sci-hub.sidesgame.com/10.1016/j.jip.2018.02.010
Delfinado, M.D. and Baker, E.W. (1961). Tropilaelaps, a new genus of mite from the Philippines (Laelapidae [s. lat.], Acarina). Fieldiana Zoology 44(7):53–56. https://www.biodiversitylibrary.org/partpdf/36189
Delfinado, M.D. (1963). Mites of the honeybee in South-east Asia. Journal of Apicultural Research2(2):113–114. https://www.wellesu.com/10.1080/00218839.1963.11100070
Delfinado-Baker, M. (1982). New records for Tropilaelaps clareae from colonies of Apis cerana indica. American Bee Journal 125(5):382.
Delfinado-Baker, M. and Baker, E.W. (1982). A new species of Tropilaelaps parasitic on honey bees. American Bee Journal 122(6):416-417.
Delfinado-Baker, M., Rath, W. and Boecking, O. (1992). Phoretic bee mites and honeybee grooming behavior. International Journal of Acarology 18(4):315-322. https://www.wellesu.com/10.1080/01647959208683966
Delfinado-Baker, M. (1994). A harmless mite found on honeybees Melittiphis alvearius: From Italy to New Zealand. American Bee Journal 134(3):199.
Direct Science (accessed 5 February 2025). Tropilaelaps. https://tropilaelaps.info/https://www.smithsonianmag.com/science-nature/beekeepers-seek-save-honeybees-colony-invading-pest-180973241/
Engelhardt, T. (8 March 2025). Beekeepers Association of New South Wales Southern Branch Varroa field day, Epic Exhibition Park, Mitchell, ACT.
Garvey, K. (1981). The giant tick. In Tales of my Uncle Harry. ABC Books, Sydney.
Gill, M. (2025). Bee Improvement and Bee Breeders Association. Tropilaelaps. https://bibba.com/tropilaelaps/
Gill, M.C., Chuttong, B., Davies, P., Etheridge, D., Panyaraksa, L., Tomkies, V., Tonge, G. and Budge, G.E. (2024). Assessment of the efficacy of field and laboratory methods for the detection ofTropilaelaps spp. PloS ONE 19(9):p.e0301880. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0301880
Hoppe, H., Ritter, W. and Stephen, E.W.C. (1989). The control of parasitic bee mites: Varroa jacobsoni, Acarapis woodi and Tropilaelaps clareae with formic acid. American Bee Journal129(11):739-742. https://scholar.google.com.au/scholar?hl=en&as_sdt=0%2C5&q=+The+control+of+parasitic+bee+mites%3A+Varroa+jacobsoni%2C+Acarapis+woodi+and+Tropilaelaps+clareae+with+formic+acid.&btnG=
Janashia, I., Uzunov, A., Chen, C., Costa, C. and Cilia, G. (2024). First report on Tropilaelaps mercedesae presence in Georgia: The mite is heading westward!. Journal of Apicultural Science68(2):183-188. https://intapi.sciendo.com/pdf/10.2478/jas-2024-0010
Johnson, B.R. (2023). Honey Bee Biology, Chapter 16, Tropical honey bees – Varroa resistance, pp.293-295. Princeton University Press.
Jordan, M. and Rogers, S. (5 November 2024). Current distribution of Tropilaelaps mercedesaeassociated with Apis mellifera honey bees. Auburn University. https://www.honeybeepests.org/tropi-distribution
Khongphinitbunjong, K., Neumann, P., Chantawannakul, P. and Williams, G.R. (2015). The ectoparasitic mite Tropilaelaps mercedesae reduces western honey bee, Apis mellifera, longevity and emergence weight, and promotes deformed wing virus infections. Journal of Invertebrate Pathology 137:38-42. https://sci-hub.sidesgame.com/10.1007/s13592-015-0407-5
Kochansky, J. and Shimanuki, H. (1999). Development of a gel formulation of formic acid for control of parasitic mites of honey bees. Journal of Agricultural and Food Chemistry 47(9):3850-3853. https://www.wellesu.com/10.1021/jf9901439
Koeniger, N., Koeniger, G. and Delfinado-Baker, M. (1983). Observations on mites of the Asian honeybee species (Apis cerana, Apis dorsata, Apis florea). Apidologie 14(3):197-204. https://www.apidologie.org/articles/apido/pdf/1983/03/Apidologie_0044-8435_1983_14_3_ART0005.pdf
Mahmood, R., Wagchoure, E.S., ul Mohsin, A., Raja, S. and Sarwar, G. (2012). Control of ectoparasitic mites in honeybee (Apis mellifera L.) colonies by using thymol and oxalic acid. Pakistan Journal of Zoology 44(4):985-989. https://www.academia.edu/57831568/Control_of_Ectoparasitic_Mites_in_Honeybee_Apis_mellifera_L_Colonies_by_Using_Thymol_and_Oxalic_Acid
Martin, S.J. and Correia-Oliveira, M.E. (2016). The occurrence of ecto-parasitic Leptus sp. mites on Africanized honey bees. Journal of Apicultural Research 55(3):1–4. https//doi:10.1080/00218839.2016.1228214
Melissocosmos (accessed 25 March 2025). Tropilaelaps mite. https://melissocosmos.blogspot.com/2022/03/tropilaelaps-mite-echthros-melisswn.html
Namin, M.S., Joharchi, O., Aryal, S., Thapa, R., Kwon, S.H., Kakhramanov, B.A. and Jung, C. (2024). Exploring genetic variation and phylogenetic patterns of Tropilaelaps mercedesae(Mesostigmata: Laelapidae) populations in Asia. Frontiers in Ecology and Evolution 12:1-8. https://www.frontiersin.org/journals/ecology-and-evolution/articles/10.3389/fevo.2024.1275995/full
Ochoa, R., Pettis, J.S. and Mireles, O.M. (2003). A new bee mite of the genus Pseudacarapis (Acari: Tarsonemidae) from Mexico. International Journal of Acarology 29(4):299-305. https://doi:10.1080/01647950308684345
Oldroyd, B.P. and Wongsiri, S. (2009). Asian honey bees: Biology, conservation, and human interactions. Harvard University Press.
Oliver, R. (2024). Randy Oliver’s latest work, October 22, 2024: A New York Bee Wellness webinar https://youtu.be/LtNB04jfER4?list=TLPQMTgxMjIwMjSR9hO7fJ3FLg
Oliver, R. (March 2025) pers comm.
Pettis, J.S., Rose, R. and Chaimanee, V. (2017). Chemical and cultural control of Tropilaelaps mercedesae mites in honeybee (Apis mellifera) colonies in Northern Thailand. PloS ONE, 12(11):p.e0188063. https://journals.plos.org/plosone/article/file?id=10.1371/journal.pone.0188063&type=printable
Poorna Chandra, S., Srinivas Reddy, K., Chinnamade Gowda, C. and Eswarappa, G. (2021). Mites associated with honey bee colonies with special reference to phoretic mite, Neocypholaelaps indicaEvans in selected locations of Southern Karnataka. Journal of Entomology and Zoology Studies9(1):629-635. https://www.entomoljournal.com/archives/2021/vol9issue1/PartI/8-6-281-653.pdf
Raffique, M.K., Mahmood, R., Aslam, M. and Sarwar, G. (2012). Control of Tropilaelaps clareae mite by using formic acid and thymol in honey bee Apis mellifera L. colonies. Pakistan Journal of Zoology44(4):1129-1135. https://www.academia.edu/57831515/Control_of_Tropilaelaps_clareae_Mite_by_Using_Formic_Acid_and_Thymol_in_Honey_Bee_Apis_mellifera_L_Colonies
Ramsey, S. (2024). Tropilaelaps mites—A fate worse than Varroa. Notes from annual conference of the American Beekeeping Federation. https://phillybeekeepers.org/notes-from-annual-conference-of-the-american-beekeeping-federation/
Roberts, J.M., Anderson, D.L. and Durr, P.A. (2017). Absence of deformed wing virus and Varroa destructor in Australia provides unique perspectives on honeybee viral landscapes and colony losses. Scientific Reports 7(1):6925. https://www.nature.com/articles/s41598-017-07290-w.pdf
Roberts, J.M., Schouten, C.N., Sengere, R.W., Jave, J. and Lloyd, D. (2019). Different strategies to manage Varroa jacobsoni and Tropilaelaps mercedesae in Papua New Guinea. BioRxiv 2019-12. https://www.biorxiv.org/content/biorxiv/early/2019/12/19/2019.12.17.880393.full.pdf
Roberts, J.M.K., Schouten, C.N., Sengere, R.W., Jave, J. and Lloyd, D. (2020a). Effectiveness of control strategies for Varroa jacobsoni and Tropilaelaps mercedesae in Papua New Guinea.Experimental and Applied Acarology 80(3):399-407. https://doi:10.1007/s10493-020-00473-7
Roberts, J.M., Simbiken, N., Dale, C., Armstrong, J. and Anderson, D.L. (2020b). Tolerance of honey bees to Varroa mite in the absence of deformed wing virus. Viruses 12(5):575. https://sci-hub.sidesgame.com/10.3390/v12050575
Rubink, W.L., Delfinado-Baker, M., Wilson, W.T., Gonzalez-Gracia, M.D.L. and Gonzalez-Cortés, S. (1991). A phoretic uropodid mite associated with honey bee swarms of northeastern Mexico. International Journal of Acarology 17(4):259-263. https://www.wellesu.com/10.1080/01647959108683916
Southcott, R.V. (1989). A larval mite (Acarina: Erythraeidae) parasitizing the European honey bee in Guatemala. Acarologia 30(2):123-129. https://www1.montpellier.inrae.fr/CBGP/acarologia/article.php?id=2522
The National Bee Unit (2017). Tropilaelaps parasitic mites of honey bees. https://www.nationalbeeunit.com/assets/PDFs/3_Resources_for_beekeepers/Advisory_leaflets/Tropilaelaps_2017_web_version_.pdf
Travel online: Cook Islands (accessed 5 February 2025). https://www.travelonline.com/cook-islands/tours/island-hopper-vacations/nightlife/party-bus-42027.jpg
Uzunov, A., Gabel, M. and Büchler, R. (2023). Summer brood interruption for vital honey bee colonies: Towards sustainable Varroa control using biotechnical methods. Apoidea Press.
Uzunov, A., Gabel, M. and Büchler, R. (2025). Summer brood interruption: Why and how. The Beekeepers Quarterly 159:17-19.
Wilson, W.T., Woolley, T.A., Nunamaker, R.A. and Rubink, W.L. (1987). An erythraeid mite externally parasitic on honey bees (Apis mellifera). American Bee Journal 127:853-854.
Wilson, W.T., Rubink, W.L. and Collins, A.M. (1990). A larval species of erythraeid mite (Leptus sp., Acarina: Erythraeidae) ectoparasitic on adult honey bees (Apis mellifera L.) in south Texas. Bee Science 1(1):18-22. https://www.cabidigitallibrary.org/doi/full/10.5555/19910229801
