
Alan Wade
The Lord will smite thee with the botch of Egypt,
and with the haemorrhoids,
and with the scab, and with the itch,
whereof thou canst not be healed.
Deuteronomy 28:2
There is considerable disquiet amongst natural and many side-line beekeepers at the prospect of being persuaded to employ synthetic miticides to control the Varroa scourge. They have heard about mites developing resistance to such pesticides but are anyway averse to persistent chemicals ending up in comb wax or in the honey they sell to their customers.
However a number of effective mite suppression schemes, ones designed to avoid the use of these hard chemicals, have been developed and are widely employed in Europe and North America. They range from mite trapping and forcing brood breaks, to dequeening, to shook swarming (see Part I) and to use of natural products that are more lethal to mites than to bees. These control measures are reviewed here as Part II while we will go further and explore the use of emerging products, largely unrefined natural plant extractives and biological control agents in Part III.
The organic mite slayers
The most commonly employed natural treatment products are terpene derived products and simple organic acids (Figure 1).
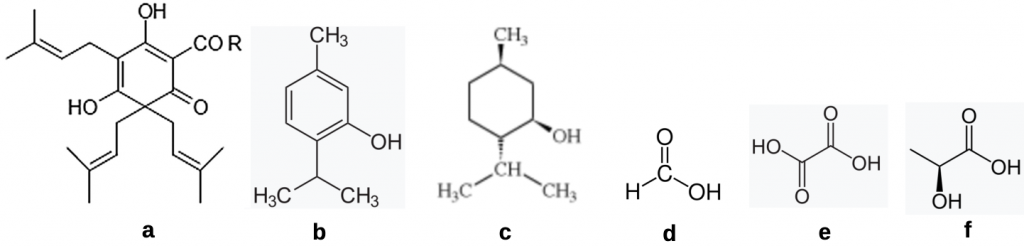
Figure 1 Natural products commonly employed for treating mites:
Terpenes
(a) β hop acids [R = CH2CH(CH3)2, lupulone; R = CH(CH3)2, colupulone; R = CH(CH3)CH2CH3, adlupulone];
(b) thymol [5-methyl-2-(propan-2-yl)phenol];
(c) (-)-menthol [methyl-2-(propan-2-yl)cyclohexan-1-ol];
Organic acids
(d) formic acid [methanoic acid];
(e) oxalic acid [ethanedioic acid]; and
(f) lactic acid [2-hydroxypropanoic acid].
Practical mite treatment
Alternative remedies to both the synthetics and the standard knockdown organics are to be found in Randy Oliver’s extended oxalic acid (OAE) and another product already in use, extended formic acid (FAE). They have received minimal attention. They remove Varroa mites over at least one complete brood cycle targeting mites developing under protective brood cell wax cappings. A further strategy is to impregnate the likes of cardboard strips to effect continuous slow release of natural oils and indeed products as volatile as formic acid that extend mite kill for many weeksi.
Let’s first look the treatment products already in wide use for mite control recognising that they too each have their strengths and shortcomings. No natural product has been reported to result in any form of mite resistance though there is no reason to believe that this cannot occur. This is not to say that natural product use should not be rotated as this is the common recommendation for the use of synthetic miticides where resistance has developed remarkably quickly. The main downside of employing natural products is that they are nearly all simply quick knockdown agents. Mites residing inside wax covered cells, predating on developing pupae, are protected and are unaffected by most natural agents, formic acid being the notable exception. It targets all mites, the acid molecule being small enough in size to permeate the wax cappings.
Terpenes
Broadly speaking the natural organics can be categorised as terpenes (Figure 1: a,b,c) built on the five-carbon building block isoprene (Figure 2) or simple organic acids (Figure1:d,e,f). However, in the absence of well designed trials it may be wise to hold off using any of the of essential oils known to kill mites until their efficacy and potential to impact adversely on bees has been evaluated under field conditions.
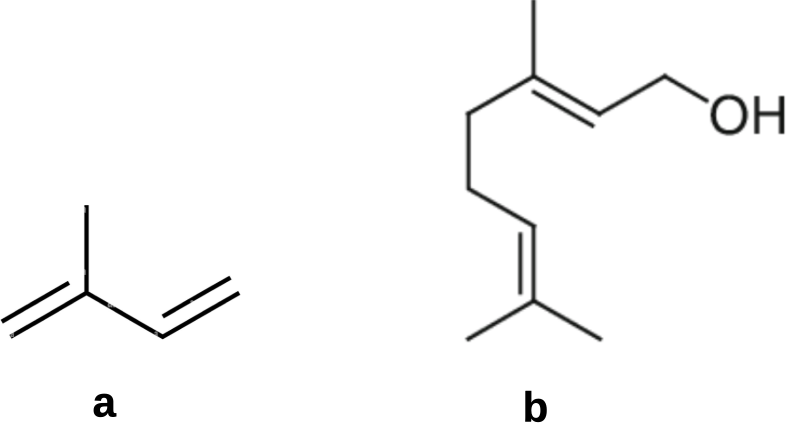
Figure 2 Basic terpene building blocks:
(a) simple five-carbon building block, isoprene (2-methyl-1,3-butadiene); and
(b) common intermediate monoterpene geraniol ((2E)-3,7-dimethyloctan-2,6-dien-1-ol).
Simple terpenes are either liquids or low melting point solids. A number of natural oils, principally terpenes, appear to achieve a high mite kill and with correspondingly low bee mortality. However most have a very short term action, measurable in a time period of days, so ony target phoretic mites, not those under brood cell cappings.
Colin and coworkersii have shown the thymol, apart from directly killing mites, promotes uncapping and removal of mite affected larvae, a hygienic behaviour that appears to change over the course of repeated treatment. Thymol interferes with 4-amino butyric acid receptors that mediate both bee and mite neural behaviour. How thymol, and indeed the many more common aliphatic terpenes, actually kill phoretic mites is by no means certain.
(a) The β hop acids
The β hop acids (Figure 1a), with isoprene side chains, are major components of the hop bush (Humulus lupulus) that give beer much of its flavour and aromaiii. The most recent commercial mite treatment formulation, HopGuard®3, comprises the potassium (phenolate) salts of the lupulonesiv: these β acids are obtained by selectively extracting hop resins with propyline glycol [1,2-propanediol]. Unlike many muscular beekeepers, bees are averse to the lupulones: with both bees and people one is never quite sure:
He had his beer from year to year;
And then his bier had him.
Nevertheless the lupulones are very effective in removing mites on contact and do not affect brood or honey so can be used when honey supers are in place. HopGuard® 3 is applied at the rate of one strip per five brood framesv. Its action is short-lived, about three days, so a second treatment is required to knock down emerging mites. The product is yet to become commercially available in Australia, but its use and effectiveness versus more conventional arachnicides was reviewed recently by Kim and coworkersvi.
A wide range of hop oil chemical constituents has been documented by Iglesias and coworkersvii. They comprise terpenes, bitter acids – including the active β hop acids, chalcones, flavonol glycosides and catechins. However the terpene fraction (Figure 3) is only 1-2% of the oil mix so their presence is unlikely to be responsible for mite kill.
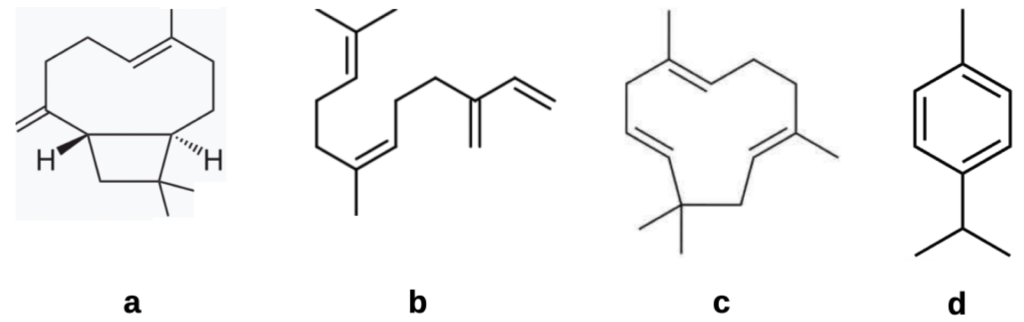
Figure 3 Main sesquiterpene and monoterpene hop oil components:
(a) β-caryophyllene;
(b) farnesene:
(c) humulene: and
(d) myrcene.
(b) Thymol
Thymol (Figure 1b), an aromatic plant metabolite of terpenoid origin and a major component of thyme, is a scheduled miticide. It is widely available either as an easy to apply gel (Apiguard®) or as a white solid readily available as a plant extractive. It has gained some popularity as a late season treatment where little brood is present. It is a slowly evaporating crystalline solid and is typically applied at a rate of 50 g per hive where it acts over a period of 2-4 weeks. Since it will taint honey it can only be applied after the honey crop has been removed. Its use is compared with that of oxalic acid by Mahmood and coworkersviii, a helpful perspective since both are well suited to late season mite control.
(c) Menthol
Menthol (Figure 1c) is a monoterpene alcohol – a waxy solid – found in mint family plants such as peppermint. The natural laevorotatory (anticlockwise light bending) (-)-enantiomer melts at 36-38 0C while the synthetic racemic mix is produced by hydrogenating thymol melts at 42-45 0C.
As a miticide, it has been used in stand alone treatment at a rate of 50 g per hive, or sometimes in conjunction with wintergreen oil (spearmint; methyl salicylate) or with tea tree oil where menthol is found with other terpenes and β-triketones. It has been more widely applied admixed with thymol, eucalyptol and camphor (see Figures 1b,c and 4a,b) formulated as ApilifeVAR®ix. There are limited records of eucalyptol (1,8-cineol)x – the common primary component of Eucalyptus oils and the widely harvested cajuput oil (from Melaleuca leucadendron and Melaleuca cajuputi) – and camphorxi (camphor laurel, Camphora officinarum) being employed as stand alone varroacides, the likely effectiveness of the commercial product may be attributed to its major constituent thymol.
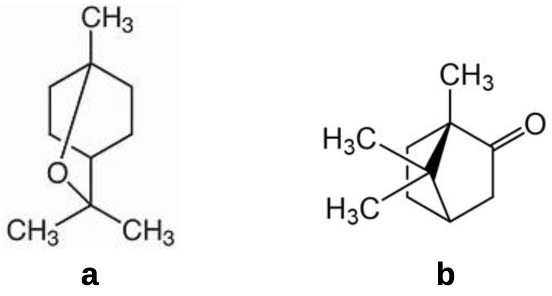
Figure 4 A sampler of other terpenes found in mixes employed to treat mites:
(a) eucalyptol [1,8-cineole; 1,3,3-trimethyl-2-oxabicyclo[2.2.2]acetate)]; and
(b) S-camphor [1,7,7-trimethylbicyclo[2.2.1]heptan-2-one]
Organic acids
The principal mode of action of the organic acids is respiratory acidosis to which mites succumb. Their key detraction is that the free acids inflict some damage to brood and adult bees, and may result in premature queen supersedure. For this reason scheduled requeening is often conducted after treatment.
(d) Formic acid
Formic acid treatment
This acid, the simplest of all organic acids (Figure 1d), permeates wax cappings and is effective in killing both phoretic (roaming mites) and those mites parasitising developing pupae. However it will also kill some bees, particularly eggs and larvae, so its application requires a modicum of care. Ideally it is best applied to broodless bees or when there is sealed brood only. In any case it should not be used when the ambient air temperature is above 30 0C.
While some ventilation is needed to avoid high bee mortality – hive entrances need to be fully open – screened bottom boards and any vents need to be closed to ensure the formic acid vapour circulates through the brood nestxii. It is a little too harsh to be employed in nucleus colonies. Because formic acid is so volatile and since it is steadily released from a specially designed envelope its action is limited to a short period after the package is opened. Typically two strips are applied and treatment is completed in a 14 day period.
Note that formic acid has a low boiling point (100.8 0C) and is fuming liquid at room temperature. While purchasable in sealed ampules, it is both unsafe to handle and is unsuitable to use in the pure form.
FormicProTM is supplied as a wrapped formate ester-polysaccharide (i.e. an esterified starch) gelxiii that is snipped to begin formic acid release when inserted above brood frames.
The commercial product is formed by reacting formic acid with a sugar polymer under anhydrous conditions. In the process formate esters of 6-glucose units of the polysaccharide are formed (Figure 5)xiv. When the product is exposed to air it absorbs moisture and hydrolyses releasing free formic acid.
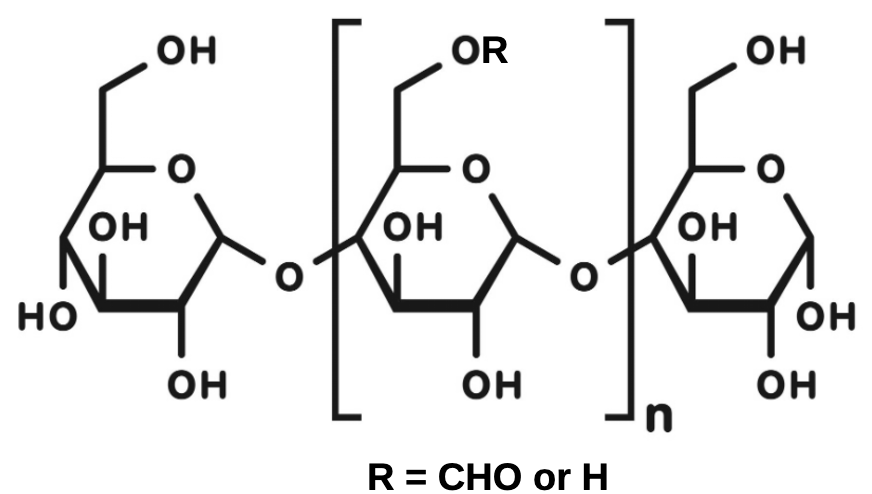
Figure 5 Starch glucose-6-formate esters formed by reacting formic acid with a polysaccharide (a starch polymer). Web template redrawn by Alan Wade
Extended formic acid (FAE)
FormicPro can be also be used in an extended application. In standard application two strips are applied to brood frames in a single 14 day treatment. In extended application single strips are applied sequentially (at a 10 day interval) to give a 20 day treatment. Formic acid is eventually exhausted obviating the need to remove the packaging. The US manufacturer, NOD Apiary Products, claims that FormicPro gives a 83-97% mite killxv. A 2002 Argentinian trialxvi, employing a slow release formic acid gel (carboxipolimetilen or carboxypolymethalene, a carboxyvinyl polymer, admixed with formic acid), is reportedly similarly successful.
(e) Oxalic acid
Oxalic acid treatment
In normal and widespread use, oxalic acid (Figure 1e) can be applied as a syringe-able dilute aqueous dribble (or spray). It is particularly effective in a fogging application, where oxalic acid is sublimed. The gear required for sublimation is expensive and full protective gear including respirator PPE is needed: we recommend it not be used.
Oxalic acid (unless sublimated) has a different action to that of formic acid. Mites are only targeted if they come into physical contact with the acid, so its key application is to treat hived swarms or broodless bees.
The simplest (also cheapest) option is to syringe in 5 mL of a medium strength aqueous solution of the acid between each brood frame. The standard acid concentration (formally approved) is a 3.2% (32 g/L) solution made up by first dissolving the acid in 1:1 sugar syrup and diluting it with water (Table 2). Oxalic acid is also available in kit makeup form as Api-BioxalTM and as AluenCAP® but they are more expensive than common pure wood bleach. However made up the solution has a limited shelf life due to microbial oxidation.
| Oxalic acid strength | Hot mix 4.2% w/v | Medium mix 3.2 % w/v | Weak mix 2.5% w/v |
| OA crystals (dihydrate) | 60 g | 45 g | 35 g |
| Water | 600 mL | 600 mL | 600 mL |
| Sugar | 600 g | 600 g | 600 g |
Table 2 Standard makeup of dribble oxalic acid after Oliverxvii.
Notes: Makes 1 L stock solution to treat ~ 20 hives. A hot mix is employed for very high mite infestations while the medium mix is used for normal colony treatment. The weak mix is used in cold weather prior to a broodless period.
Alternatively Oliver recommends dissolving oxalic acid in the common food grade glycerine (in lieu of sugar syrup) claiming it adheres better to bees so is more effective (Table 3).
| Oxalic acid strength | Standard 3.2 % w/v |
| OA crystals (dihydrate) | 45 g |
| Glycerine | 50 mL |
| Water | 1000 mL |
Table 3 Sticky glycerine makeup for dribble oxalic acid treatment.
The defining features of oxalic acid is its quick knockdown action. Within a few days, however, that acid is completely lost as it degrades quickly. Its fate is likely facilitated by the enzyme oxalate oxidase found in plants, wood and fungixviii.
Extended oxalic acid (AOE)
Admixed with pure glycerine, oxalic acid is retained and is released slowly resulting in protracted mite kill, its mode of action replicating that of persistent synthetic miticides. A perplexing question is the role of glycerine in facilitating the slow release of the acid. The Oliver-style oxalic-glycerine mix is formulated under conditions not dissimilar to those described by Gladys and coworkersxix and is also indicated closer to room temperature by Alksnis, Gruziń and Surnaxx. Glycerine reacts with oxalic acid when heated to form mono and di-esters (Figure 6) but oxalic acid disproportionates, in lay parlance disintegrates, if overheated.
The di-ester is formed more rapidly than the mono-ester so seems likely to be a major component of the mix. However the ratio of oxalic acid to glycerine (on a mole for mole basis) based on the Randy Oliver OA strip formulation only exceeds that of glycerine by a factor of 1.1:1 so both esters will be present.
Under ambient conditions, and exposed to air or to moist brood nest conditions, the prepared strips gradually release oxalic acid as the esters hydrolyse.
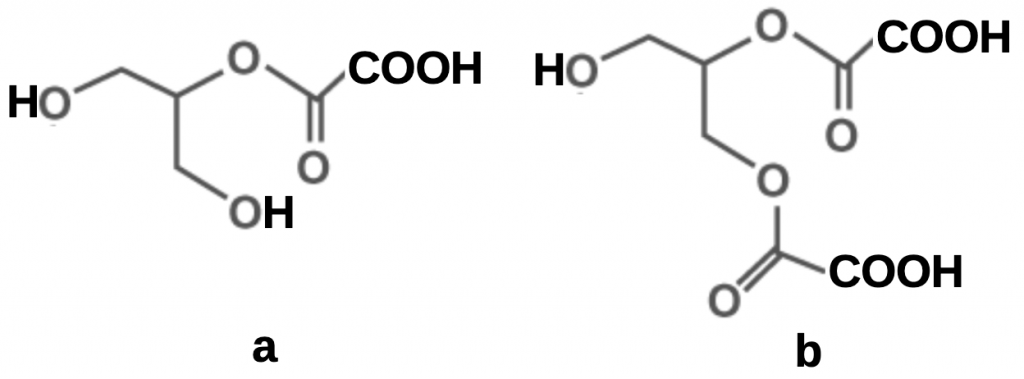
Figure 6 Oxalic acid esters of glycerine (1,2,3-trihydroxypropane):
(a) oxalate mono-ester; and
(b) oxalate di-ester.
Web template redrawn by Alan Wade
In a preliminary trial, conducted in the Newcastle district and replicating the Oliver procedurexxi, AOE strips were applied to rescue apparently healthy hives with high mite loads, those that were adjudged at risk of imminent collapse.
Extended oxalic acid (EOA) treatment strips can be made from pure wood bleach (oxalic acid dihydrate) and food grade glycerine impregnated into highly absorbent dishcloths (Table 4):
| Treatment strip makeup | Quantity | Molar equivalent |
| Oxalic acid dihydrate | 500 g | 3.25 |
| Glycerine | 400 mL | 2.88 |
| Dishcloth strips | 22 strips (9×22 cm2) |
Table 4 Swedish dishcloth strips makeup for extended oxalic acid treatment.
Notes: The density of glycerine is 1.26 g/mL, so the weight to weight ratio of pure materials is close to 1:1. The molar (i.e chemical) ration of oxalic acid to glycerine is 1.13.
Oxalic acid is stirred into the glycerine in a saucepan and heated gently ensuring that the maximum temperature does not exceed 65 0C. The mixture is poured over strips in a deep receptacle (such as an old bread baking tin) turning over strips with tongs and laying saturated strips on an old sheet of tin to cool. Any small amount of residual liquid can be mopped us with a spare absorbent strip that can be used to treat a strong nucleus hive.
Wear gloves and goggles, use tongs to handle the acid mix and have a bucket of water with a few tablespoons of sodium bicarbonate at the ready to mop up spills and to wash hands. Swedish dishcloths are well known for their absorptive capacity and are cellulose based so can be composted after use if not removed by the bees themselves. Other absorptive media such as egg carton bases may suffice.
Strips, once cool, must be promptly stored in airtight sealable plastic bags (or airtight tubs) as glycerine is hydroscopic. Note that strips may end up sticky though Randy Oliver reports that some oxalic acid present will crystallise, but will redissolve when placed on top of brood next frames with relative humidity of around 80%. Rusty Berlewxxii provides simple instructions for making up a few OAE strips also based on the original formulation provided by Randy Oliverxxiii.
To use the prepared pads, lay two strips (containing an equivalent of 45 g of free oxalic acid) well spaced across central frames between double brood boxes, that is laid over bottom brood nest frames. While I have not heard of OAE strips being used in single brood box operation, it is important to ensure there is bee space above the treatment strips to provide nurse bees their full access. The treatment can be scaled down if there are few frames of brood.
Oliver reports that oxalic acid – however applied – is broken down very quickly in the hive so will not produce detectable residue above natural background levels found naturally in honey.
Using a half-cup (~ 300) of nurse bees for a standard alcohol wash in the Newcastle trial, mite levels were reduced from 150 to 28 (84%) over a seven week period. The procedure can be enhanced by moving the queen to a small nucleus colony where, brood free, it can be dribble treated with normally formulated 3.2% oxalic acid. The main colony, made queenless and raising its own queen, is then subjected to extended oxalic acid treatment. Mites released by progressively emerging brood will be fully exposed to oxalic acid and the colonies can be reunited and, if needed, requeened after treatment.
New advances in oxalic acid treatments
Strips of a commercial variant of an oxalic acid-glycerine formulation, Aluen Cap, were trialled in an extended 42 day Argentinian study by Maggi and coworkersxxiv, They reporting that this product gave good Varroa control and could be used over the summer period in the presence of brood. It was followed by another study of Aluen Cap conducted on Africanised Apis mellifera honey beesxxv also conducted in Argentina.
Kanelis and coworkersxxvi have very recently reported on an optimal optimum oxalic acid-glycerine formulation (21% (w/v): 60 g OA, 130 mL glycerine and 100 mL water absorbed onto absorbent strips) that achieves a 90.4% to 94.5% mite kill rate without adversely affecting bee brood. Sabahi and coworkers have also compared the relative performance of OAE with standard stand alone oxalic acid, thymol and oregano oil (Table 5)xxvii though they observed some bee mortality associated with the use of oxalic acid formulations.
| Treatment agent | Formulation medium | Mean mite mortality (%) |
| Control | 18 | |
| Thymol | dust | 96.6 |
| Thymol-glycerine | 92.4 | |
| Oregano oil | microcapsules | 21.3 |
| Oregano oil-glycerine | 69.1 | |
| Oxalic acid | dust | 20 |
| Oxalic acid-glycerine | towelling | 79 |
Table 5 Four week autumn trial comparing efficacy of various non-synthetic agents applied weekly [ascertained by mite fall].
Note: Most mites were killed in the first two weeks.
Kosch, Mülling and Emmerichxxviii have now completed a systemic review of various oxalic acid formulations, including those containing glycerine, concluding that there is little evidence of Varroa mites developing any resistance to oxalic acid.
(f) Lactic acid
Lactic acid (2-hydroxypropanoic acid; Figure 1f) is an inexpensive and effective miticidexxix but may result in high egg loss and should not be used with honey supers on since it can impart honey a sour taste. Like thymol it is an ideal product to employ for that critical autumn or early winter treatment to reduce mite loads. It is normally available commercially as an 80% aqueous solution – the pure acid has a melting point of 18 0C (boiling point 122 0C). Miscible with water it is easy to handle but presumably difficult to obtain water free. Like the other organic acids it has a short effective half life once applied to brood nests.
Since lactic acid is a contact miticide it is sprayed directly onto bees and at a rate of 5-6 mL (15% lactic acid) per brood frame face. Treatments need to be repeated 2-4 times per year with a recommendation to conduct repeat treatments coming into winter. The claimed mite mortality rate is 83-99% though some reports suggest a kill rate as low as around 80%xxx.
In concluding Part III In search of the mitey sledgehammer, we will examine emerging techniques for mite control, the promising mite fungal pathogens and an array of natural oils that kill mites under controlled laboratory conditions. However they are only now beginning to be fully evaluated for their miticide efficacy in the hive.
Readings
iBoonmee, T., Sinpoo, C., Wongthaveethong, L., Disayathanoowat, T., Suanpoot, P., Pettis, J.S. and Chaimanee, V. (2024). Properties of essential oils absorbed on the surface of cardboard pieces after using atmospheric-pressure plasma treatments to develop long-lasting Varroa miticides in honeybees (Apis mellifera). PLOS One 19(2):p.e0297980 https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0297980
iiColin, T., Lim, M.Y., Quarrell, S.R., Allen, G.R. and Barron, A.B. (2019). Effects of thymol on European honey bee hygienic behaviour. Apidologie 50():141-152. https://link.springer.com/content/pdf/10.1007/s13592-018-0625-8.pdf
iiiDanenhower, T.M., Force, L.J., Petersen, K.J., Betts, T.A. and Baker, G.A. (2008). HPLC analysis of 𝛂-and 𝛃-acids in hops. Journal of Chemical Education 85(7):954-956. doi:10.1021/ed085p954
Krofta, K. and Mikyška, A. (2014). Hop beta acids: Properties, significance and utilization. Kvasný Průmysl 60(4):96-105. https://doaj.org/article/b5ccec45fde0452cac99876089cdf122
ivKostrzewa, D., Dobrzyńska-Inger, A., Rój, E., Grzęda, K. and Kozłowski, K. (2016). Isomerization of hop extract α-acids. Journal of the Institute of Brewing 122(3):493-499. doi:10.1002/jib.349
vOliver, R. and Roberts, A. (2022). Hopguard 3 as a summer treatment. Scientific Beekeeping. https://scientificbeekeeping.com/hopguard-3-as-a-summer-treatment/
viKim, K.M., Park, B.S., Kim, J.G., Kang, E.J., Choi, Y.S. and Kim, D.W., (2022). Toxicological assessment of major acaricides for controlling ectoparasitic mites of the honey bee, Apis mellifera, on the honey bee workers. Journal of Apiculture 37(1):1-5. https://pmc.ncbi.nlm.nih.gov/articles/PMC6023343/
viiIglesias, A., Mitton, G., Szawarski, N., Cooley, H., Ramos, F., Arcerito, F.M., Brasesco, C., Ramirez, C., Gende, L., Eguaras, M., Fanovich, A. and Magi, M. (2020). Essential oils from Humulus lupulus as novel control agents against Varroa destructor. Industrial Crops and Products 158:113043. https://doi.org/10.1016/j.indcrop.2020.113043
viiiMahmood, R., Wagchoure, E.S., ul Mohsin, A., Raja, S. and Sarwar, G.(2012). Control of ectoparasitic mites in honeybee (Apis mellifera L.) colonies by using thymol and oxalic acid. Pakistan Journal of Zoology 44(4):985-989. https://zsp.com.pk/pdf44/985-989%20_13_%20PJZ-736-11%20revised%20version%20of%20article.pdf
ixAdamczyk, S., Lázaro, R., Pérez-Arquillué, C., Conchello, P. and Herrera, A. (2005). Evaluation of residues of essential oil components in honey after different anti-Varroa treatments. Journal of Agricultural and Food Chemistry 53(26):10085-10090. doi:10.1021/jf051813f
xBrasesco, C., Gende, L., Negri, P., Szawarski, N., Iglesias, A., Eguaras, M., Ruffinengo, S. and Maggi, M. (2017). Assessing in vitro acaricidal effect and joint action of a binary mixture between essential oil compounds (thymol, phellandrene, eucalyptol, cinnamaldehyde, myrcene, carvacrol) over ectoparasitic mite Varroa destructor (Acari: Varroidae). Journal of Apicultural Science 61(2):203-215. https://intapi.sciendo.com/pdf/10.1515/jas-2017-0008
xiHarris, S. (1 December 2017). Varroa biology and management: An overview. University of Florida blogs. https://blogs.ifas.ufl.edu/entnemdept/2017/12/01/varroa-biology-management-overview/
xiiAnderson, C. (2024). Formic acid treatment for bees. Carolina Honeybees. https://carolinahoneybees.com/formic-acid-treatment-for-bees/
xiiiUnited States Environmental Protection Agency (2019). Letter of approval for NOD Apiary Products FormicPro for treatment of varroosis caused by Varroa destructor in honey bees (Apis mellifera). https://www3.epa.gov/pesticides/chem_search/ppls/075710-00003-20190711.pdf
xivDivers, T., Pillin, I., Feller, J.-F., Levesque, G. and Grohens, Y. (2004). Starch modification, destructuration and hydrolysis during O-formylation. Starch-Stärke 56(9):389–398. doi:10.1002/star.200300270
xvUnited States Environmental Protection Agency (2019) loc. cit.
xviEguaras, M., Palacio, M.A., Faverin, C., Basualdo, M., Del Hoyo, M.L., Velis, G. and Bedascarrasbure, E. (2003). Efficacy of formic acid in gel for Varroa control in Apis mellifera L.: Importance of the dispenser position inside the hive. Veterinary Parasitology 111(2-3):∫41-245. https://www.wellesu.com/https://doi.org/10.1016/s0304-4017(02)00377-1
xviiOliver, R. (2023). Oxalic acid treatment table. Scientific Beekeeping. https://scientificbeekeeping.com/oxalic-acid-treatment-table/
xviiiGrąz, M., Ruminowicz-Stefaniuk, M. and Jarosz-Wilkołazka, A. (2023). Oxalic acid degradation in wood-rotting fungi: Searching for a new source of oxalate oxidase. World Journal of Microbiology and Biotechnology 39(13): doi:10.1007/s11274-022-03449-4
xixGladys, H.P., Cho, S.K., Yeong, Ooi, T.L. and Chuah, C.H. (2006). Glycerol esters from the reaction of glycerol with dicarboxylic acid esters. Journal of Surfactants and Detergents 9(2):147-152. doi:10.1007/s11743-006-0384-9
xxAlksnis, A.F., Gruziń, I.V. and Surna, Y.A. (1976). Synthesis of oligoesters from oxalic acid and glycerol. Journal of Polymer Science: Polymer Chemistry Edition 14(11):2631-2638. https://www.deepdyve.com/lp/wiley/synthesis-of-oligoesters-from-oxalic-acid-and-glycerol-re1EagOR5a
xxiOliver, R. (2022a). Extended-release oxalic (OAE) update Part 1. Scientific Beekeeping. https://scientificbeekeeping.com/7701-2/
Oliver, R. (2022b). Extended-release oxalic (OAE) update Part 2. Scientific Beekeeping. https://scientificbeekeeping.com/2022-extended-release-oxalic-oae-update-part-2/
xxiiBerlew, R. (2017). How to use oxalic acid & glycerin strips for varroa. Honey Bee Suite. https://www.honeybeesuite.com/oxalic-acid-and-glycerin-for-varroa-mites/
xxiiiOliver, R. (2022a; 2022b) loc. cit.
xxivMaggi, M., Tourn, E., Negri, P., Szawarski, N., Marconi, A., Gallez, L., Medici, S., Ruffinengo, S., Brasesco, C., De Feudis, L. and Quintana, S., (2016). A new formulation of oxalic acid for Varroa destructor control applied in Apis mellifera colonies in the presence of brood. Apidologie, 47:596-605. https://link.springer.com/content/pdf/10.1007/s13592-015-0405-7.pdf
xxvRodríguez Dehaibes, S.R., Meroi Arcerito, F.R., Chávez-Hernández, E., Luna-Olivares, G., Marcangeli, J., Eguaras, M. and Maggi, M. (2020). Control of Varroa destructor development in Africanized Apis mellifera honeybees using Aluen Cap (oxalic acid formulation). International Journal of Acarology 46(6):405-408. Paper accesible at Google Scholar.
xxviKanelis, D., Tananaki, C., Liolios, V., and Rodopoulou, M. (2023). Evaluation of oxalic acid with glycerin efficacy against Varroa destructor (Varroidae): A four year assay. Journal of Apicultural Research63(5):847-855. https://doi.org/10.1080/00218839.2023.2169368
xxviiSabahi, Q., Morfin, N., Emsen, B., Gashout, H.A., Kelly, P.G., Otto, S., Merrill, A.R. and Guzman-Novoa, E. (2020). Evaluation of dry and wet formulations of oxalic acid, thymol, and oregano oil for varroa mite (Acari: Varroidae) control in honey bee (Hymenoptera: Apidae) colonies. Journal of Economic Entomology 113(6):2588-2594. https://academic.oup.com/jee/article/113/6/2588/5916618?login=false
xxviiiKosch, Y., Mülling, C. and Emmerich, I.U. (2024). Resistance of Varroa destructor against oxalic acid treatment: A systematic review. Veterinary Sciences 11(9):393. https://pdfs.semanticscholar.org/17ba/c73d39a304d49b74d4a421031ee650bca90a.pdf
xxixMAF Biosecurity (accessed 14 August 2024). A review of treatment options for control of Varroa mite in New Zealand. HortResearch Client Report No. 2001/249. https://www.bobsbeekeeping.com.au/image/bee-resources/varroa-treatment-options.pdf
Imdorf, A., Charriere, J.D, Maquelin, C., Kilchemann, V. and Bachofen, B. (1996). Alternative Varroa control. Agrarforschung 3(4):173-176. https://agris.fao.org/search/en/providers/122607/records/6471f8062a40512c710f0b49
xxxIbrahim, S.H. and Ezzat, T.H. (1993). Zur wirkung von milchsäure auf den Varroa-befall der biene. [Studies on the effect of lactic acid on Varroa infestation of the bee.] Anzeiger fur Schadlingskunde, Pflanzenschutz, Umweltschutz 66(2):31-32. doi:10.1007/BF01909139
