
Bap the Baker
Alan Wade
Banging on for a Better Solution
In Part I we surveyed the parlous state of bees and their keepers taken to their limit by the mighty mite. What we need is visionary souls, like Bap the Baker, to lead the way forward:
Then Bap the Baker leaped to his feet
And cried, ‘What do wasps like best to eat?
Strawberry jam! Now wait a minute!
If we made a giant sandwich we could trap them in it!’
The gentlemen cheered, the ladies squealed,
And Farmer Seed said, ‘Use my field.’
Bap gave instructions for the making of the dough.
‘Mix flour from above and yeast from below.
Salt from the seaside, water from the spout.
Now thump it! Bump it! Bang it about!’
So far we have tried to set down reasons why control of Varroa mites has proved to be exceptionally difficult. Faced with a losing battle to control the pesky invaders with synthetic miticides, North American and European beekeepers and researchers have turned to approaches that native Asian bee hosts of Varroa employ in the wild. They have also looked to natural product remedies and honey bee breeding to crack the nut of mite control and to these we turn to here.
In practice we need to kill mites, interrupt their reproduction – using mite trapping or biotechnical (aka brood cycle interruption) means – or suppress mite and virus viability. An elusive solution is to have the bees equip themselves to tolerate Varroa while also keeping the bees productive and other pests and diseases at bay.
Let’s start with an approach that employs treating bees with natural products. These include simple organic acids and natural – largely terpene-based – oils and low melting point solids.
Scheme 3 Natural product knock down strategy
There are a range of well-tested natural products that are lethal to mites (Figure 1). Their action is mainly of short duration and, unlike synthetic miticides that may be left in the colony for a month or (unwisely) more, most plant extractives are simply knock down agents. But how effective are these natural products and what needs to be done to ensure the mites, and not sensitive brood, are effected by these agents? While their action is quick and sharp, measures such as admixing oxalic acid with glycerine impregnated into strips can extend their treatment efficacy.
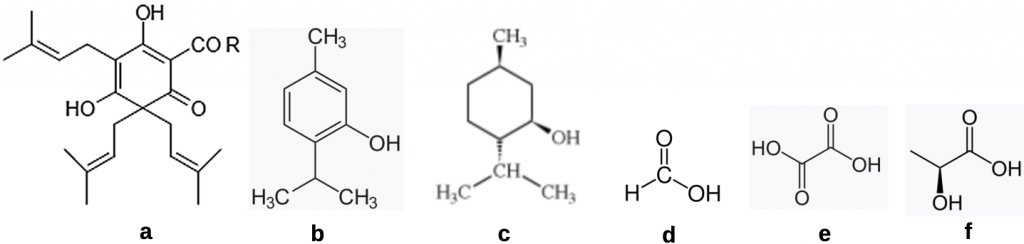
Figure 1 A menagerie of natural products for treating mites:
(a) β hop acidsi;
(b) thymol;
(c) menthol;
(d) oxalic acid;
(e) formic acid; and
(f) lactic acid.
Of these natural products, oxalic acid (found in oxalis, rhubarb and spinach), formic acid (think bull ants), thymol (a terpene found in thyme), hop β acids (α and b hop acids are the bitter principles of beer) and lactic acid are the best tested products.
There is general consensus that mites are unlikely to build up resistance to the types of natural plant metabolites employed. For beekeepers, ever keen to find a home made solution to any malady, the small amounts of these chemicals found in rhubarb leaves, thyme bushes, half a cup of ants or in a sky reaching beer hop vine will not produce enough material to constitute an effective dose. Popping a few rhubarb leaves or crushed ants under a hive lid simply won’t work. As a rule of thumb stick to formulations approved for use and glove and mask up as recommended.
It is important to realise that despite your best efforts you will still encounter colony losses, so try to requeen bees heavily infested with mites – with the best available stock – or euthanase weak colonies especially those badly co-infected with the likes of chalk brood and Nosema. And if you are as averse as I am to using synthetic miticides then judicious use of natural products and adopting techniques such as removal of drone comb as it is sealed are strategies for reducing Varroa that can be extended into honey flows. The drone producing season depends of course on where you live, so a mix of interventions will always be needed.
Scheme 4 Natural defence strategy
It was only some time after western honey bees encountered long mite-tolerant eastern honey bees that the Korean and the Japanese-Thai halotypes of Varroa destructor, those parasitising Apis cerana, jumped ship to our honey bees. When the mites came up the tights came down. So started the Varroa epidemicii.
The natural hosts of parasitic bee mites – there are about a dozen such blood suckers (see for example Figure 2) – are intrinsically mite tolerant and have been so for millennia. In the long term only those mites that allowed their Apis species hosts to survive have themselves survived.
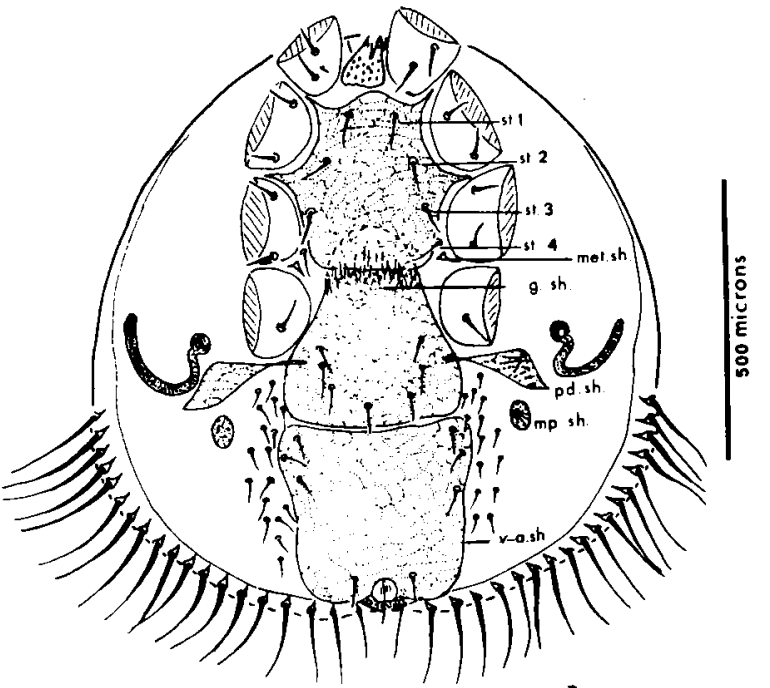
Figure 2 Euvarroa sinhaiiii, a mite hosted by Apis florea: bee and mite were co-established recently on the Burrup Peninsula in Western Australia.
Notice the drawing shows legs surgically removed.
While Mossadegh reports Euvarroa sinhai feeding, living and reproducing on regular honey bees to which mites were introducediv, Koeniger and coworkers signal that these mites do not survive long when caged with Apis mellifera and are usually absent from hives found sympatric with Apis melliferav. Nevertheless they are occasionally found in floor debris of mellifera coloniesvi.
From this Ben Oldroydvii has surmised that since Apis mellifera colonies have long been kept in close proximity to Apis florea they are unlikely to cross over. The greater risk, he suggests, is that Apis florea will become widely established and itself adversely impact our bee industry.
We now know that parasites of Asian honey bees all harbour viruses but, with their native hosts restricting the number of mites present, their virus loads and the mites are only occasionally lethalviii. The subtleties and range of factors occasioning colony collapse due to viruses in western honey bees are outlined by Carreck, Ball and Martinix.
Against this background of freeloading mites what might we learn from bees themselves? In a short time, less than 100 years, a number of western honey bee populations have come up with the defences that all their Asian cousins acquired over many millennia. Le Conte and coworkersx identify some of the many pockets of global Varroa tolerance amongst western honey bees and identify the mechanisms by which they have achieved that level of resilience. Lockexi similarly identifies specific regional mite-resistant biotypes systematically describing each population. Others have described Varroa-tolerant populations in specific regions, Rozenkranz in South Americaxii, Oddie, Dahle and Neumann in Norwayxiii, Rinderer and coworkers in eastern Russiaxiv, Fries and coworkers in a Nordic climatexv and Riley in Irelandxvi. By and large, however, Varroa tolerance has not translated to such bees being successfully introduced elsewhere, well not in the sense that once established that tolerance is lost when queens are superseded or colonies swarm. As Le Conte and coworkers have surmised:
Since the emergence of Varroa as a serious pest of Apis mellifera, considerable time, effort and finance has been devoted to understanding the mechanisms underlying varroa resistance and to breeding bees resistant to the mite. However, progress has often been slow, and some desirable traits, demonstrable in experimental colonies, show low heritability or, alternatively, show benefits that are too small to render them practicable in breeding programs. Another problem is that bee populations apparently resistant to varroa in one location sometimes cease to remain resistant when moved elsewhere and exposed to different environmental conditions or exposed to different mite populations.
Brian Johnson makes a case for a rather more nuanced understanding of the inherent Varroa resistance of Africanised honey bees in the central Americasxvii. Based on genetics and behavioural observations, he attributes much of the resilience to vigorous grooming behaviour, to very effective mite dismemberment, to very effective patrol and removal of mite infested brood and to often shortened brood raising time (~18.5 days for African races versus 20-21 days for European races of the species). He also tracks the changing behaviour of bees in central American apiaries stocked with European bees surrounded by wild colonies – Africanised drones mating with European queens – with a resultant shift in genetics. In any case Africanised honey bees appear to result in the production of low fertility mites, hypothesised to be caused by higher activity of bee larvae that results in high mortality of the sole male mite that is laid first.
Johnson observes that bees in Brazil suffer essentially no varroa damage while there is notable if slight varroa damage to bees in Mexico. Brazilian bees host the less pathogenic Varroa jacobsoni mite and this was originally hypothesised to explain the between country difference in varroa susceptibility. However Varroa destructor is now in Brazil and the impact has not changed so that theory has been jettisoned.
If we add in the increased swarming proclivity and smaller nests of African bees we might begin to conclude that African and European races of honey bees are distinct Apis mellifera ecotypes subject to very different environmental influences. Johnson’s overall thesis is that European and African bees are best adapted to cooler and tropical climes respectively, but only those of African origin are intrinsically Varroa tolerant.
This backgrounding leads us back to the topic of intervention, measures that might be adopted to reduce mite loads. Particularly effective are the techniques for separating bees from their mite laden brood outlined by Uzunov, Gabel and Büchlerxviii and by others such as Randy Oliverxix and Kirsty Staintonxx. Their ploys can entirely circumvent use of any chemicals or only require their judicious use. All these procedures (apart from treating swarmed bees upon capture) require radical interruption to the brood cycle so are difficult to adopt on a commercial scale. The techniques amount either to confining the queen to allow brood to mature and emerge to force mites to become phoretic – where mites are exposed and can be more effectively treated – or to physically separating bees from their brood – as in shook swarming.
The downside to techniques that mimic natural defence are those of an intensive labour requirement and the loss of brood raising often attendant to queen caging. Techniques that keep the colony queen laying can, however, be used to advantage. In these queens are encouraged to lay out drone comb, mites being preferentially attracted to their brood, that comb being cycled out promptly once it is sealed, in any case well before drones emerge. Such techniques would appear to be sustainable but require close attention by the beekeeper making them best suited to the sideline beekeeper.
Scheme 5 Honey bee mite tolerant strategy
Enter a new generation of queen breeders who are making slow but steady progress towards raising Varroa tolerant stock (see for example van Alphen and coworkersxxi). Specific traits, often routinely observable, have been targeted: auto and allogrooming, brood uncapping, mite dismemberment, healthy brood recapping… Selection for several or all such traits must pass a certain threshold for mite removal to be effective.
Harking back to the ‘do nothing scenario’ we discover that our honey bees have latent genes, often poorly expressed, that enable the fittest colonies to survive. Once selected for and retained we might just get sustainably mite tolerant and productive bees. For those sceptical of this eventuality, breeders are now producing stock with a modicum of chalkbrood and other brood disease hygienic behaviour.
Implicit to this optimism are the many efforts to raise Varroa tolerant bees: the USDA’s program to employ Primorsky (eastern Russian) mite tolerant Apis mellifera stock, the Arista program in Hawaiixxii, the New World Carniolan projectxxiii, the UK Westerham project and the Randy Oliver projectxxiv. Few queens from such programs are available commercially and those that are attracting a premium price. By way of example queens from the Oliver stable are only now just being offered for sale through commercial breeders such as Nature’s Nectarxxv. They report that:
The Olivers don’t select for looks and use their queens’ variation in color patterns as an indicator that they are maintaining enough genetic diversity in their stock. But they do select for performance.
They breed only from queens whose colonies are very gentle and healthy, exhibited above average honey production, and proved strong for almond pollination.
Westerham Beekeepers, and seemingly others, have been similarly successful in operating apiaries with carefully selected stock, albeit mainly for their own use. Their scheme, to operate their bees entirely treatment free, has been seventeen years in the making.
Are Australian beekeepers and queen breeders able to do their bit? This is hard to say. However buying the cheapest rather than the best – if shopping at the supermarket is any indication – is the norm. The Australian Queen Bee Breeders Association (AQBBA), if not the broader beekeeping industry and queen producers, are gearing up to produce Varroa tolerant stock both to counter the spread of the mite and to deal with problems of high colony losses that will ensue once the mites become well established.
Established way back in the 1980s, AQBBA’s mission has been to breed productive, disease resistant and tractable stock. From a pool of 500 high performance stock from New South Wales and Queensland apiaries, queens from ten colonies demonstrating a useful level of Varroa tolerance (Unhealthy Bee Odour tested) have now been distributed to AQBBA breeders along the eastern seaboard. These untested but ideally mated queens are now being used to raise stock that are being assessed for Varroa Sensitive Hygiene performance. The coordinated program is in its earliest stages of development, some queens raised being tested in the Varroa infested Newcastle area.
The exigencies of breeding and the likely future establishment of regionally stable Varroa resistant populations is some while off. For now, the alternative to intensive mite management will be to purchase not-so-cheap queens whose progeny, also selected for productivity and gentleness, have a modicum of Varroa tolerance.
This all said, the question remains as to whether science and beekeeping practice will ever match the Varroa tolerance already achieved by African bees and pockets of surviving wild VSH bees. Have these bees been smarter in finding their own solution? After all the denizens of Itching Down eventually found their own solution to an invasive pest.
Suddenly the sky was humming!
All four million wasps were coming!
They smelled that jam, they dived and struck!
And they ate so much that they all got stuck.
The other slice came down – kersplat! –
On top of the wasps, and that was that.
My bet is on the AQBBA achieving this lofty goal, not on spending most of my time keeping mites in check.
Readings
iKostrzewa, D., Dobrzyńska-Inger, A., Rój, E., Grzęda, K. and Kozłowski, K. (2016). Isomerization of hop extract α-acids. Journal of the Institute of Brewing 122(3):493-499. doi:10.1002/jib.349
iiCarreck, N. and Neumann, P. (2010). Honey bee colony losses. Journal of Apicultural Research 49(1):1-6. doi:10.3896/IBRA.1.49.1.01
Berthoud, H., Imdorf, A., Haueter, M., Radloff, S. and Neumann, P. (2010). Virus infections and winter losses of honey bee colonies (Apis mellifera). Journal of Apicultural Research 49(1):60-65. doi:10.3896/IBRA.1.49.1.08
iiiAkratanakul, P. and Burgett, M. (1976). Euvarroa sinhai Delfinado and Baker (Acarina: Mesostigmata): A parasitic mite of Apis florea. Journal of Apicultural Research 15(1):11-13. doi:10.1080/00218839.1976.11099826
ivMossadegh, M.S. (1990). Development of Euvarroa sinhai (Acarina: Mesostigmata), a parasitic mite of Apis florea, on A. mellifera worker brood. Experimental and Applied Arachology 9(1-2):73-78. doi:10.1007/bf01198984
vKoeniger, N., Koeniger, G., De Guzman, L.I. and Lekprayoon, C. (1993). Survival of Euvarroa sinhai Delfinado and Baker (Acari, Varroidae) on workers of Apis cerana Fabr, Apis florea Fabr and Apis mellifera L in cages. Apidologie 24(4):403-410. doi:10.1051/apido:19930407 https://hal.science/hal-00891085/document
viKapil, R.P. and Aggarwal, K. (1987). Euvarroa sinhai found in Apis mellifera hive debris. Bee World 68(4):189-190.
Rath, W. and Delfinado-Baker, M. (1990). Analysis of Tropilaelaps clareae populations from the debris of Apis dorsata and Apis mellifera in Thailand. In Proceedings of the International Symposium on Recent Research on Bee Pathology, September 5-7, 1990, Ghent, Belgium. pp.86-89. https://www.cabidigitallibrary.org/doi/full/10.5555/19920232168
viiOldroyd, B.P. (personal communication).
viiiOldroyd, B.P. and Wongsiri, S. (2006). Asian Honey Bees. Harvard University Press. Chapter 9 Parasites, pathogens, predators and a plant, pp.180-208.
ixCarreck, N.L., Ball, B.V. and Martin, S.J. (2010). Honey bee colony collapse and changes in viral prevalence associated with Varroa destructor. Journal of Apicultural Research 49(1):93-94. doi:10.3896/IBRA.1.49.1.13
xLe Conte, Y., Meixner, M.D., Brandt, A., Carreck, N.L., Costa, C., Mondet, F. and Büchler, R. (2020). Geographical distribution and selection of European honey bees resistant to Varroa destructor. Insects 11(12):873–. doi:10.3390/insects11120873 https://www.semanticscholar.org/reader/7021c98640747071e4c9d588a052319ca546f3ef
Le Conte, Y., de Vaublanc, G., Crauser, D., Jeanne, F., Rousselle, J.-C. and Bécard, J.-M. (2007). Honey bee colonies that have survived Varroa destructor. Apidologie 38(6):566-572. doi:10.1051/apido:2007040
xiLocke, B. (2016). Natural Varroa mite-surviving Apis mellifera honeybee populations. Apidologie 47(3):467-482. doi.org/10.1007/s13592-015-0412-8
xiiRosenkranz, P. (1999). Honey bee (Apis mellifera L.) tolerance to Varroa jacobsoni Oud. in South America. Apidologie 30(2-3):159-172. doi.org/10.1051/apido:19990206
xiiiOddie, M.A., Dahle, B. and Neumann, P. (2017). Norwegian honey bees surviving Varroa destructor mite infestations by means of natural selection. PeerJ 5:p.e3956. https://peerj.com/articles/3956/
xivRinderer, T.E., de Guzman, L.I., Delatte, G.T., Stelzer, J.A., Lancaster, V.A., Kuznetsov, V.I.C.T.O.R., Beaman, L., Watts, R. and Harris, J.W. (2001). Resistance to the parasitic mite Varroa destructor in honey bees from far-eastern Russia. Apidologie 32(4):381-394. doi.org/10.1051/apido:2001138
Rinderer, T.E. and Coy, S.E. (2020). Russian honey bees. Salmon Bayou Press.
Collison, C. (2023). A closer look: Russian honey bees. The Australasian Beekeeper 124(12):46-48.
xvFries, I., Imdorf, A. and Rosenkranz, P. (2006). Survival of mite infested Varroa destructor honey bee Apis mellifera colonies in a Nordic climate. Apidologie 37(5):564-570. doi:10.1051/apido:2006031
xviRiley, S. (2024). The honey bee solution to Varroa: A practical guide for beekeepers. Northern Bee Books, Scout Bottom Farm, Mytholmroyd, West Yorkshire.
xviiJohnson, B.R. (2023). Honey Bee Biology. Chapter 16, pp.290-300. Princeton University Press.
xviiiUzunov, A., Gabel, M. and Büchler, R. (2024). Summer brood interruption for vital honey bee colonies: Towards sustainable Varroa control using biotechnical methods. Apoidea Press, Woodside Cottage, Dragons Lane, Shipley, West Sussex UK.
xixOliver, R. (December 2023). Oxalic acid treatment table. Scientific Beekeeping. https://scientificbeekeeping.com/oxalic-acid-treatment-table/
Oliver, R. (April 2009). The learning curve – Part 1: Killing mites without killing your bees. Progress report. Scientific Beekeeping. https://scientificbeekeeping.com/the-learning-curve-part-1-2009-progress-report/
Oliver, R. (May 2009). The learning curve – Part 2: Killing mites without killing your bees. Scientific Beekeeping. https://scientificbeekeeping.com/the-learning-curve-part-2-killing-mites-without-killing-your-bees/
Oliver, R. (May 2015). The learning curve – Part 3: The natural miticides. Scientific Beekeeping. https://scientificbeekeeping.com/the-learning-curve-part-3-the-natural-miticides/
Oliver, R. (2022). Extended-release oxalic (OAE) udate Part 1. Scientific Beekeeping. https://scientificbeekeeping.com/7701-2/
Oliver, R. (2022). Extended-release oxalic (OAE) udate Part 2. Scientific Beekeeping. https://scientificbeekeeping.com/2022-extended-release-oxalic-oae-update-part-2/
Berlew (2017). Honey Bee Suite. How to use oxalic acid & glycerin strips for varroa. https://www.honeybeesuite.com/oxalic-acid-and-glycerin-for-varroa-mites/
xxStainton, K. (2022). Varroa management: A practical guide on how to manage Varroa mites in honey bee colonies. Northern Bee Books. Scout Bottom Farm, Mytholmroyd, West Yorkshire.
xxivan Alphen, J.J.M. and Fernhout, B.J. (2020). Natural selection, selective breeding, and the evolution of resistance of honeybees (Apis mellifera) against Varroa. Zoological Letters 6(1):6–. doi:10.1186/s40851-020-00158-4
xxiiArista Bee Research: Documentary of Brandpunt Varroa resistant breeding project on Hawaii. (May 2018). https://aristabeeresearch.org/
xxiiiApis Information Resource Centre (accessed 31 May 2024). Sue Cobey and the New World Carniolan Breeding Program. https://beekeep.info/a-treatise-on-modern-honey-bee-management/genetic-management/sue-cobey-and-the-new-world-carniolan-breeding-program/
xxivOliver, R. (September 2023). Selective breeding progress report 2023. Scientific Beekeeping. https://scientificbeekeeping.com/selective-breeding-progress-report-2023/
xxvNature’s Nectar (15 January 2024). Introducing the Randy Oliver golden western queens! https://www.naturesnectarllc.com/introducing-the-randy-oliver-golden-west-queens/
