Alan Wade

If ever there were a cringeworthy national anthem, the original version of Advance Australia Fair would have to take the cake. Scribed in 1879 by Scottish composer Peter Dodds McCormick,i it reminds us that we are an island girt by sea:
When gallant Cook from Albion sailed,
To trace wide oceans o’er,
True British courage bore him on,
Til he landed on our shore.
Then here he raised Old England’s flag,
The standard of the brave;
“With all her faults we love her still”
“Britannia rules the wave.”
Honey bees have been in Australia for just two hundred years and could only have ever got here by human agency. Two centuries on, we brought its nemesis Varroa destructor to our shores. Will we ever see minted coins to herald new mite arrivals?
Not only but also
What must we do to protect our bees from such ‘illegal’ immigrants? Firstly we must acknowledge that the mites are here to stay and that synthetic miticides are not the once touted long-term solution to their control. US researcher-beekeeper Randy Oliver tells us that the use of these hard chemicals is not only selecting for populations of mites that are resistant to treatment but that they are also providing cover for development of a more virulent mite-vectored virus–Varroa relationship. Miticides have been chosen to minimise their effect on bee health and cognition but inevitably some synthetics end up in honey and beeswax. Randy tells us we need to move up the ladder a notch and breed bees that demonstrate natural Varroa resistance, not resistance to synthetic arachnicides. Why spend money on monitoring their inevitable spread when the obvious need for funding to develop mite resistant bees could largely resolve the problem?
But getting such hardy bees is proving to be fiendishly difficult. Strains of bees selected for resistance to Varroa have been linked to recessive genes where open mating inheritance of hygienic behaviour is quickly lost. On small isolated islands that hygienic behaviour may be retained but inbreeding results in loss of colony vigour. Wild stocks that have developed resistance appear to similarly lose that immunity when translated to new more open locations: there desirable traits such as high queen fecundity and gentleness are potentially lost.
What to do
Anna Curracan, editor of The Australasian Beekeeper, tells us where we should start. Her mantra? ‘Monitor, monitor, monitor’.ii We need to know when we have a ‘mitey’ problem, not when it is far too late to intervene.
A second sound piece of advice comes from an experienced and wily beekeeper, Ian Wallis, a long time Australian National University researcher in animal behaviour. His counsel?: ‘We will need to manage our bees extremely closely if we are to expect our bees to give us honey and pollinate our orchards and vegetables and not succumb to the mite’.
Nor should we ignore advice emanating from departments of agriculture and from key beekeeping agencies such as the Australian Honey Bee Industry Research Council. Amongst the mountains of information coming out of the woodwork, Varroa spread heat maps, reporting requirements to move bees and preemptory warnings about the ravages of the mites, we need practical guidance on how best to actually manage Varroa.
Any instructions we get will need to be clear, inexpensive, timely and free of onerous reporting requirements. We need regulators and knowledgeable beekeepers to work in sympathy and with a degree of urgency. Otherwise beekeepers will resort to what US bee guru Randy Oliver has euphemistically termed ‘unapproved use’ of agricultural miticides.iii He claims that the industry was only saved by resorting to less than legal and uncontrolled use of unregulated chemicals. To add insult to injury, he also notes that:
…beekeepers are highly tempted to use readily available off-the-shelf natural treatments (formic, oxalic, thymol and other plant oils) that present no unreasonable risk to man or the environment and would be of little concern to inspectors, but due to EPA’s [Environment Protection Authority] stringent requirement to rigidly follow their FIFRA [US Federal Insecticide, Fungicide, and Rodenticide Act] mandate, are not legally allowed.
This is not to say that the Australian experience could not be different or that our American and European counterparts on the ground have been anything other than diligent and proactive in managing Varroa and Acarapis mites. They, our overseas counterparts, have worked out what chemicals synthetic and natural work and what do not. Randy Oliver uses natural products in rotation, has a disciplined breeding program and culls lost cause colonies. His podcasts and articles truely inform good practice but we shall come back to these.
On the other side of the Atlantic there are as many qualified voices, ones we might learn from. Amongst these, Kirsty Stainton, a researcher with a pedigree in bee pest control, has produced an eminently practical managment guide.iv Her book, Varroa management: A practical guide on how to manage Varroa mites in honey bee colonies explains in the very simplest of terms the basics of mite biology and how to manage and treat the parasite. She outlines a three-pronged approach to managment:v
1 Monitor regularly for mite infestation levels.
Use the half cup of bees alcohol wash routine backed up by regular sticky mat checks.
2 Treat promptly whenever mite levels exceed the mite count threshold.
A count of >3 mites/100 bees (1/2 cup approximates 300 bees) signals a critical infestation level where treatment should be immediately initiated.
3 Adopt additional control measures that reduce mite numbers:
- use sticky mats under screened bottom boards to capture dislodged mites (a) to prevent their reentering the breeding cycle and (b) to routinely monitor mite numbers. Stainton stresses that sticky mat surveillance is not a definitive or quantitative infestation assessment technique, but it can usefully signal a change in mite numbers;
- cage queens periodically to break the mite brood cycle and hence to interrupt their reproduction. Queens on a single frame in a queen excluder cage will allow all colony brood to emerge and allow the queen to remain in a laying condition. Caging queens may only be practical in small scale operations. Under ideal conditions a good queen laying at an optimal rate of 1800 eggs per day, 1 egg every 48 seconds, will lay out both faces of a perfectly drawn out worker comb (containing 6300 worker cells) in 3.5 days so may need regular swapping out. In practice the queen need only be confined for one brood cycle;
- remove sealed drone brood on a 21 day cycle in spring and early summer. Either use starter strip combs in a normal full depth frame or insert a shallow frame and allow the bees to draw out drone comb below the bottom bar. Then either freeze frames to kill mites or scrape off drawn bottom bar drone comb every three weeks since drones emerge at day 24. A key advantage of using drone brood removal is that it affords a method of mite control when honey supers are on colonies and when treatment is least feasible;
- split hives strategically using the swap and queen transfer techniques advocated by Wally Shaw/Lois Edward Snelgrove – outlined by Stainton – to disrupt natural mite reproduction (e.g. to alternatively induce broodlessness in each split while retaining the laying queen)vi; and
- consider employing other hive splitting techniques such as shook swarming that induce broodlessness.
Natural product use and effectiveness profiles
Detailed instructions for use synthetic and natural products are found at Appendix 1. Here is a summary for natural product use adopted in Europe and North America:
Oxalic acid:In the United States and Europe many beekeepers have found treatment with oxalic acid, most simply applied by dribbling between brood frames, to be one of the cheapest and most effective treatments. Two products, Api-Bioxal and Aluen CAP are being currently evaluated for registration, but this product has not yet been authorised for use in Australia.
In Europe the simplest and most common practice is to syringe 5 mL of stock 7% oxalic acid solution between each pair of brood frames doing so as evenly as possible and after removing any top brace comb. Formulations and instructions for make up and use vary according to the supplier. Commercial formulations employ oxalic acid dissolved in 1:1 sugar syrup or provide the sugar for admixing. Most formulations need to be diluted – some products come made up – to produce a stock solution containing ~ 70 g oxalic acid per litre.
It is worth noting that Oliver limits total treatment to 50 mL of a 3.2% solution of oxalic acid per colony but also employs a hot 4.2% and weak 2.5% solution based on standard dihydrate oxalic acidvii and has used glycerine in lieu of sugar to increase oxalic adherence to bees. Oliver’s wide experimentation with sponges and absorbent pads for extended application of oxalic acid, formic and thymol used in rotation, their use, mode of action of organic acids and efficacy is informative.viii
Only treat when colonies are broodless (that is in winter or in the absence of open brood) unless some brood mortality is an acceptable tradeoff for urgently needed mite control. Oxalic acid does not target
mites developing under sealed brood and is only effective if the ambient temperature is above 3 0C. It can also be usefully employed to treat captured swarms.
Employ either an open or closed bottom board, use gloves and expect treatment to be greater than 90% effective. As a guide Oliver employs oxalic acid twice per year, once at spring nuc makeup and again in autumn (also if needed at a forced brood break). Australian approval for use of oxalic acid is pending.
Formic acid: FormicPro strips have an active emergency use permit in Australia and we can assume may get permanent registration. They consist of gel strips infused with formic acid. Use two strips per brood chamber for 7 days. Formic acid treatment is unsuitable for weak colonies containing less than six frames of bees. It can be used with supers on but use with a screened bottom board and use PPE. Neat formic acid is a fuming volatile liquid but is supplied in much safer forms, e.g. as a gel. Formulations of formic acid are effective at ambient temperatures of 10-30 0C (no greater). In practice it is greater than 95% effective even with sealed brood as volatile formic acid penetrates wax cappings.
Thymol: This is product is formulated as a thymol-infused gel and is registered for use in Australia as Apiguard. Oliver employs about 25 grams of gel per colony but varies the amount depending on colony strength. Stainton recommends 50 g per hive for 14-21 days or half this amount applied sequentially over two 10 day periods. It only works well when ambient temperatures are above 15 0C. Use a closed bottom board or insert a panel above a vented bottom board screen. Unless honey is to be reserved for raising brood, remove all supers as thymol will taint honey. Thymol treatment is 80-95 % effective.
Stainton appears to be averse to the use of synthetic miticides though they can be very effective. Sensibly she does not decry their occasional use though many will be wary of mixing synthetic miticides with beekeeping operations. While natural products work well and their mode of action do not lead to any mite resistance, they may adversely affect bees when not used as directed. Appendix 2 provides details of the physical properties of the main natural products that have been widely employed for treating Varroa infestations.
Colony splitting to reduce mite impact
Hive splitting to reduce the incidence of swarming is well understood. It mimics natural reproductive colony division. Overall the queenright split will rebuild its numbers – the queen can be caged temporarily to force a completely broodless condition – while the queenless split will suffer a brood break in the time it takes it to raise a new queen. In most respects the weakened units are so depleted of bees that they abandon swarming.
The Shaw-Snelgrove techniques that Stainton instances (discussed below) tweaks the splitting process to reduce the amount of sealed brood present, a strategy that recognises that Varroa only develops under brood that is capped. Splitting is simplified where double brood chambers are employed and where the brood boxes have (for spring operation) been regularly reversed. Here illustrated are the two techniques she describes to reduce mite numbers by splitting strong colonies.
Colonies with swarm cells
Move the colony with swarm cells to be split a meter or so and place a new bottom board and an empty super on the parent hive stand (Figure 1). Transfer two frames of eggs and young larvae to the centre of the empty super after first shaking off bees in front of the moved parent hive and flank the new brood chamber with some stores and empty frames (ideally drawn comb) or follower boards. Field bees will drift from the parent hive and the new colony will immediately commence raising new queens. However, with no sealed brood and with only a small amount of developing brood, its mite population growth will have been curtailed.
The parent colony, weakened by the loss of field bees, tears down its swarm cells as only existing nurse and emerging bees will be present. At day 9 or 10 open the daughter colony and remove all developing emergency queen cells. By this time about half the colony brood will have emerged and most of the remaining brood will be sealed. At the same time open the offset parent colony, remove the queen and transfer her to the daughter colony from which emergency queen cells have been removed. The daughter colony will be queen receptive having just lost its replacement queen cells.
The parent colony, having in turn lost its queen, will now commence raising emergency queens and, in its weakened state, will not swarm. Both colonies, each set back by the initial splitting process, will now each have experienced a period of near complete broodlessness. The queen however will have kept laying albeit across two hives.
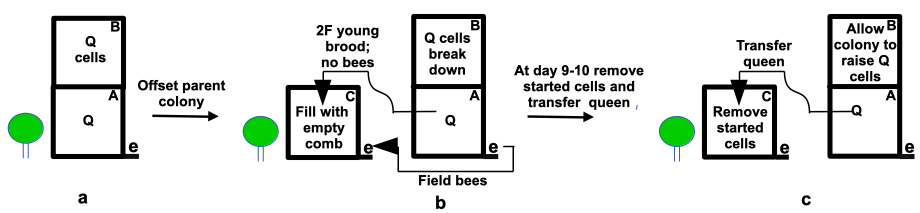
Figure 1 Splitting colony with bees preparing to swarm: e= entrance; Q = queen:
(a) colony with swarm cells;
(b) the parent colony is split after offsetting leaving only young brood and stores on the parent stand; and
(c) emergency cells started in the split on the parent stand are removed and the parent queen is transferred. The offset parent hive will now raise queen cells.
The splitting process will not eliminate mite reproduction – as a complete cycle of broodlessness will do – and of course some mites will persist, but their ability to reproduce will be severely limited. Some treatment may be in order, for example with thymol or oxalic acid.
Normal strong colonies
In this scenario, the strong colony in need of swarm control is simply split. Most brood and bees (~2/3 of colony comprising sealed brood and bees with at least one frame containing eggs and some larvae) are transferred to a new brood box and moved to a new location 3-5 km away. This leaves the parent hive with the old queen, young brood and a much reduced population on the parent hive stand (Figure 2). The parent hive has proportionally fewer mites and experiences a period where there is little sealed brood so it will be several weeks before mite numbers increase. Alternatively the queen can be caged for a complete brood cycle so the queenright split becomes fully broodless.
The daughter spit meanwhile will have the greater proportion of mites and some that will be added to the population with emerging brood. However it will take several weeks for the colony to establish an emergency queen and the new colony will experience a partial break in its brood cycle.
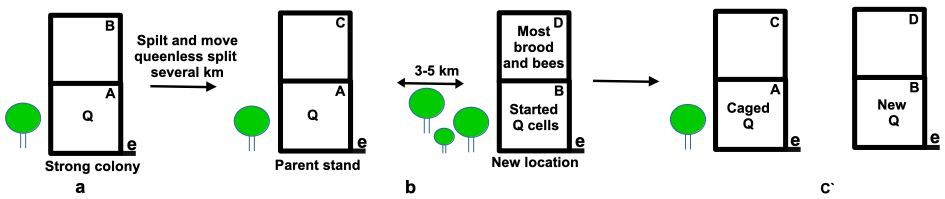
Figure 2 Simple split of a strong colony: e = entrance; Q = queen:
(a) a colony that may soon swarm;
(b) the parent colony is split on the parent stand. The bulk of bees and sealed brood together with a frame of eggs (~2/3rds of colony) are moved to a new location; and
(c) the parent colony with the caged queen will become broodless and colony with new queen, once well established, is moved back to the apiary. Both colonies now with minimal brood can be treated.
In practice it is often not be feasible to move the daughter split colony to a new location. Here field bees with their mites will drift back to the parent stand with the old queen so additional bees will need to shaken – without the queen – into the daughter colony.
Is colony splitting a check on mite reproduction?
Kirsty Stainton concludes that the effectiveness of colony splitting in controlling mite reproduction is difficult to establish conclusively. The colony split will naturally remove the swarming instinct. Note that only strong colonies, those at risk of swarming and in a condition to prevent brood chilling, should ever be split. Stainton concludes that hive splitting in combination with mite treatment is warranted.
In a follow up article Mite Bee Part II – How the Mitey have Fallen we will examine the natural defences that the original hosts of a range of honey bee mites have put up to coexist with these interlopers. We will also add to the list some further interventions that might achieve more effective mite control.
……………………………………………………………………………………………………………………………………………………….Appendix 1 Natural product treatment regimes employed in Europe and North America
| Formulation | Application | Stock solution | Dose | Conditions for use | Temp ambient | Notes | Effective-ness | Approval status |
| Natural products# | ||||||||
| Oxalic acid (Api-Bioxal) | Dribble or spray | 70 g/L in 1:1 sugar syrup | Syringe 5 mL per frame/5 sprays per side | Bees must be broodless (i.e. winter); use PPE | >3 0C | Lethal to open brood; remove brace comb | 91-95 % | Pending;approved by USDA |
| Formic acid (FormicPro) | Remove plastic packaging but not paper wrapper; widely available and easy to use | Pads soaked in 65 % formic acid (35 mL per pad) | Insert 2 strips per brood chamber) for 14 days 4-5 cm apart or 1+1 strips for 10 days in sequence located centrally on brood frames | Min 6F bees (10,000 bees); may affect or kill Q esp at higher temp; use screened bottom board; can be used with supers on; use PPE; penetrates brood cappings | 10-29.5 0C (always < 30 0C) | Can be used in summer with supers on | ~97 % | Pending (MAQS approved by USDA) |
| Thymol (Apiguard) | Trays or strips with 8-15 g thymol | 8, 12.5 and 15 g formula-tions | Use 50 g/hive; 2-4 weeks where temperature is consistently above 15 degrees [nucs, small colonies 25 g sachet] | No supers – will taint honey; no supplementary feeding; use closed bottom board. No supers, taints honey | > 15 0C | Late summer and autumn; spring only if > 15 0C [15-40 0C] | 79-97 % | Registered (2 products approved by USDA) |
| Formic acid and oxalic acid (VarroaMed) | 15-45 mL based on colony size; fairly easy to use | Syringe between frames; Multi treatments can be made at 6 day intervals; do not disturb for 7 days | Application rate 15-30 0C and 30-45 0C adjusted | ~ 90 % | Not listed | |||
| Mixed oils (Thymol variously mixed with peppermint, menthol, eucalyptol and camphor | No proven efficacy | Not listed | ||||||
| Hop β acids | Won’t penetrate capped brood | No proven efficacy | Not listed | |||||
| Synthetic miticides | ||||||||
| Flumethrin (Baverol) [a pyrethroid] Other formulations with different use specifications | 0.9 mg per strip | 4 strips per brood chamber (15-42 days); 2 strips per nuc | Cover bottom screen with wax paper or use solid bottom board | Not critical | Pending | |||
| Apistan (Tau-fluvalinate) [a pyrethroid] | 824 mg per strip | 2 strips per brood chamber (6-8 weeks) | Insert between frames 3-4 and 7-8; any time but best late summer after honey supers removed | > 10 0C | > 90 % | not registered | ||
| Amitraz (Apivar) [a formamidine] | 6.75 mg per strip | 2 strips per brood chamber (6-10 weeks); 1 strip per nuc | Insert close to brood nest, 2 frames apart, min 6 weeks. Use any time but with supers off | Not critical | 87->98 % | Registered | ||
| Coumaphos [a phophoro-thionate/ organo phosphate] | 830 mg per strip | 1strip to 5 frames bees 42-45 days | ~ 95 % | Unlikely to be registered. Banned in some EU countries | ||||
Notes: Treatments need to match seasonal and hive conditions.
Use legally registered products.
Menthol (50 g per colony for two weeks) and formic acid are registered for treatment of tracheal mite (Acarapis woodi) in the United States. Both dusting sulphur and formic acid have been effective in controlling Tropilaelaps mites.x
Flumethrin and Apistan, synthetic pyrethroids, can cause some bee mortality and like other synthetic miticides are persistent, are toxic to wildlife and to aquatic organisms. Treatment strips should not be disposed of in domestic waste.
Only treat if mite numbers exceed the 9 mites per 300 bees threshold.
Alternate treatments if using hard (synthetic) miticides to minimise mite resistance.
Consider employing hygienic stock to enhance worker grooming behaviour.
Formic acid may have adverse effect on queens.
Natural products, though naturally common enough are generally present at far too low levels to be effective for treatment: oxalic acid in rhubarb, oxalis; formic acid in ants and thymol in thyme. Rhubarb leaves for example will not control mites.
……………………………………………………………………………………………………………………………………………………….Appendix 2 Physical properties of natural products used to treat Varroa
Oxalic acid
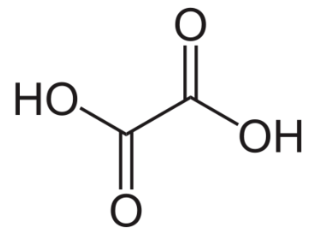
White, odourless crystalline solid (dihydrate)
Melting point: 189.5 °C
Boiling point: 365.1 °C
Density: 1.65 g/cm³
Molecular weight: 90.03 g/mol
Solubility (water): 7.4 g/l at 0 °C, 30 g/L at 14°C, 75 g/L at 40 °C, 119.4 g/L at 100 °C
Formic acid
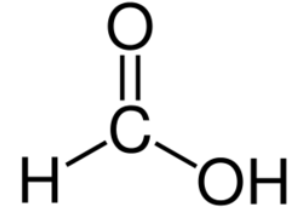
Fuming colourless liquid
Melting point: 8.4 °C
Boiling point: 100.5 °C
Density: 1.220 g/cm³
Molecular weight: 46.03 g/mol
Solubility (water): 4.09 g/l at 25 °C
Thymol
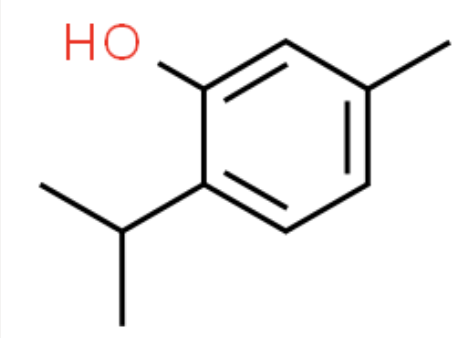
White crystalline solid with pleasant aromatic (sage) odour
Melting point: 49-51 °C
Boiling point: 232 °C
Density: 0.96 g/cm³
Molecular weight: 150.2 g/mol
Solubility (water): 0.9 g/l at 20 °C
Menthol
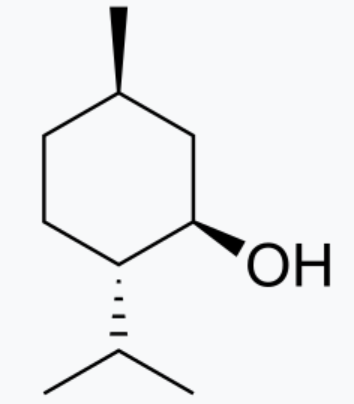
White crystallising solid with a balmy
aromatic odour
Melting point: 36-38 °C
Boiling point: 214.6 °C
Density: 0.89 g/cm³
Molecular weight: 156.3 g/mol
Solubility (water): 0.43 g/l at 20 °C
Readings
iNational Film and Sound Archive of Australia. (accessed 31 December 2023). Advance Australia fair. https://www.nfsa.gov.au/collection/curated/advance-australia-fair
iiCurracan, A. (2023). Integrated pest management: Approach to managing Varroa mites. The Australasian Beekeeper 125(6):22-25.
iiiOliver, R. (20230. The status of our industry regarding Varroa management and what to do about it. The Australasian Beekeeper 125(4):28-33.
ivFor a review of Stainton’s book see: Wade, A. (2023). Varroa management: A practical guide on how to manage Varroa mites in honey bee colonies. The Australasian Beekeeper 125(7):…
vDr Kirsty Stainton, United Kingdom Perbright Institute (2022) . Varroa management: A practical guide on how to manage Varroa mites in honey bee colonies. Northern Bee Books.Scout Bottom Farm, Mytholmroyd, West Yorkshire.
viWally Shaw (2022). An apiary guide to swarm control. Northern Bee Books. The technique varies according to whether or not swarm cells are present. It is not a treatment alternative. See also swarm control texts by Snelgrove.
viiOliver, R. (December 2023). Oxalic acid treatment table. Scientific Beekeeping. https://scientificbeekeeping.com/oxalic-acid-treatment-table/
Oliver, R. (April 2009). The learning curve – Part 1: Killing mites without killing your bees. Progress report. Scientific Beekeeping. https://scientificbeekeeping.com/the-learning-curve-part-1-2009-progress-report/
Oliver, R. (May 2009). The learning curve – Part 2: Killing mites without killing your bees. Scientific Beekeeping. https://scientificbeekeeping.com/the-learning-curve-part-2-killing-mites-without-killing-your-bees/
Oliver, R. (May 2015). The learning curve – Part 3: The natural miticides. Scientific Beekeeping. https://scientificbeekeeping.com/the-learning-curve-part-3-the-natural-miticides/
viiiOliver, R. and NY Bee Wellness (accessed 19 March 20240. Extended release oxalic, Randy Oliver- NY Bee Wellness webinar. https://youtu.be/zTuxhRDWBaw
ixPettis, J.S., Rose, R. and Chaimanee, V. (2017). Chemical and cultural control of Tropilaelaps mercedesae mites in honeybee (Apis mellifera) colonies in Northern Thailand. PLOS One 12(11):e0188063. doi:10.1371/journal.pone.0188063 https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0188063
Anderson, D.L. and Roberts, J.M.K. (2013). Standard methods for Tropilaelaps mites research. Journal of Apicultural Research 52(4):1-16. doi:10.3896/ibra.1.52.4.21 https://doi.org/10.3896/IBRA.1.52.4.21
de Guzman, L.I., Williams, G.R., Khongphinitbunjong, K. and Chantawannakul, P. (2017). Ecology, life history, and management of Tropilaelaps mites. Journal of Economic Entomology 110(2):319-332. doi:10.1093/jee/tow304 https://academic.oup.com/jee/article/110/2/319/3063341?login=false
xSammataro, D., Gerson, U. and Needham, G. (2000). Parasitic mites of honey bees: Life history, implications, and impact. Annual Review of Entomology 45(1):519-548. doi:10.1146/annurev.ento.45.1.519 https://pubmed.ncbi.nlm.nih.gov/10761588/
.
