
Alan Wade
In this episode, we delve deeper into the biology of the giant honey bees, magnificent insects, but emphatically fearsome creatures. Here we review the defining features of the known giant honey bees.
With geographic isolation, subspecies of any animal diverge enough over time to become new species. The jury is still out on the evolutionary pathway of the Giant Sulawesi Honey Bee Apis dorsata binghami. It differs in several respects from its Asian mainland Giant Honey Bee Apis dorsata dorsata sister.
The Giant Honey Bee Apis dorsata (Apis dorsata dorsata)
The general biology of The Giant Honey Bee (Apis dorsata) is broadly canvassed by Randall Hepburn and Sarah Radloffi and, in a more contemporary fashion, by Oldroyd and Wongsiriii. Paar and coworkersiii track the genetic variability and mating behaviour of north Indian populations of this bee examining the propensity of many colonies to aggregate at one location, a feature not shared by cavity dwelling bees. These bees track seasonal sources of food, governed by the phenology (seasonal pattern of blooming) of flowering plants. They might be said to have invented migratory beekeeping.
The giant honey bees – all species – are notable in their propensity to migrate rather than simply to abscond in response to dearth or disturbance. The dwarf honey bees, the Asian honey bees and many African races of Apis mellifera do likewise. From the earliest studies of the biogeography of the giant honey beeiv, it was clear that there were a second species related to Apis dorsata, the high elevation and more northerly Himalayan Honey Bee, Apis laboriosav.
The type species, Apis dorsata [Apis dorsata dorsata], migrates seasonally over vast distances, very notably some 50 km across the Melaka Straight between Sumatra and the West Malaysian peninsula and up to 200 km in Sri Lanka. On these long journeys colonies bivouac at favourable locations returning to the principle nesting site or to the shelter they had reproductively swarmed from. Apis dorsata ranges from western India, eastwards below the Himalayas, throughout continental Asia and across much of the Indonesian archipelago.
All giant honey bees occupy a single comb, mostly clustering communally. Robinsonvi as well as Underwoodvii and Woyke and Wildeviii have described the cyclical annual swarming and migratory behaviours of Apis dorsata and Apis laboriosa. Their movement patterns vary widely so that, while lower altitude populations of Apis dorsata migrate over large distances, Himalayan (Mountain) Honey Bee (Apis laboriosa) migrations are elevational and colony relocation distances are relatively short.
The overall annual pattern is one of all species of giant honey bee expanding rapidly and swarming to establish new colonies, then abandoning their nests and migrating. Robinson describes a bivouacking (staged migration) behaviour of Apis dorsata and the importance of preservation of these sites to protect the integrity of populations of this bee. These bees are famously raided for their honey stores and are important crop pollinatorsix.
All the giant honey bees, despite their propensity to migrate, can store large quantities of honey (Morse reports more than 50 kg) under favourable seasonal conditions. For example it has had a seemingly long history of being farmed for honey production on the lower Mekong River delta (Figure 6)x.
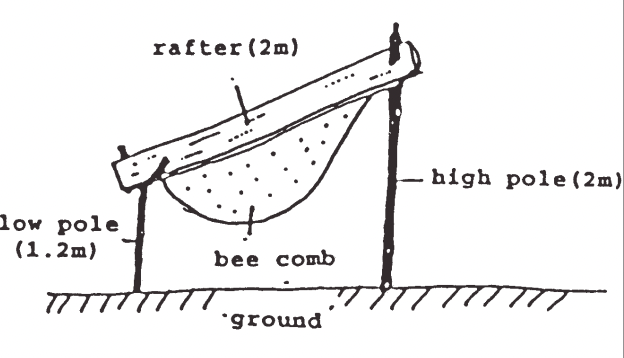
Figure 6 Apis dorsata rafter farming in the Melaleuca cajuputi forests of Vietnam
The Giant Mountain Honey Bee (Giant Himalayan Cliff Honey Bee) Apis laboriosa
Mountain dwelling Apis laboriosa is the largest of all living honey bees. Its self imposed geographic isolation, namely its adaptation to high altitude living, is both remarkable and ‘extra’ ordinary.
Initially considered to be confined to the lower slopes of the Himalaya, its natural range is now known to extend east to the mountainous region of North Vietnamxi (Figure 7). Its migratory pattern is, however, strikingly different to that of Apis dorsata. While its colonies aggregate in similarly large numbers it does so in inaccessible cliff overhangs (Figure 8) while the common and widespread giant honey bees nest under eaves of dwellings and in large trees.
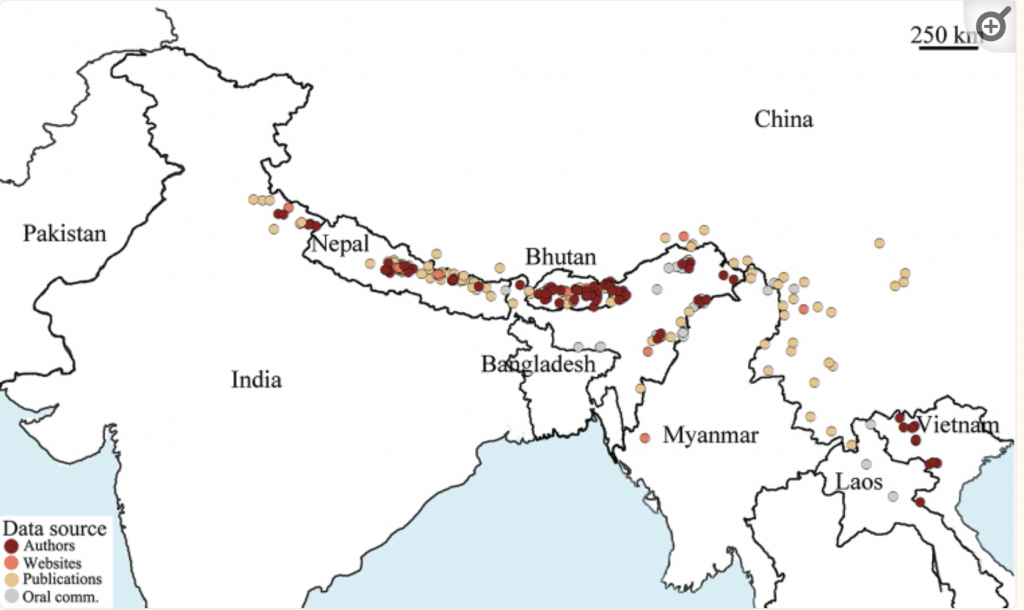
Figure 7 Giant Mountain Honey Bee distribution
The ups and downs of Apis laboriosaxii
As we have seen the altitudinal migration of Apis laboriosa is a defining characteristic of this cliff-dwelling honey bee giant. In his doctoral studies Benjamin Underwoodxiii examined the behaviour and energy balance of high-altitude survival signalling that it nests in the open, perched high on cliffs:
One of the keys to colony survival at such high altitudes is seasonal migration. Nest sites within the subalpine zone (above approximately 2800 m) are occupied for a maximum of only four months in summer (June through September), while those within the warm-temperate zone (1200 to 2000 m) may be used for as long as ten months of the year (February through November).
…a combination of poor foraging conditions and low colony stores also leads laboriosa colonies to migrate. In the steep, narrow valleys inhabited by these bees, the seasonal abundance and distribution of floral resources vary widely within short distances, and colonies need not move more than 10 to 20 km to migrate between cliff sites separated by as much as 2000 m in altitude. There they survive the cold winter months of December and January by huddling near the ground.
In this atypical – for honey bees – hibernating state they rely on camouflage and an extremely low metabolic rate to survive returning to the same lofty overhangs over summer.
Underwood further reports on the Nepalese flight performances of Apis laboriosa, Apis cerana and Apis mellifera and their ability to regulate their flight efforts in response to expected gains and associated costs as demonstrated by their being presented with different sugar level rewards.
All this means is that bees – within the limits of their physiological makeup and their seasonal propensity to migrate – adjust their behaviour so they only collect nectar if it is profitable for their colonies to do so. Underwood suggests that Apis laboriosa may be grouped with Apis dorsata and Apis florea [the common dwarf honey bee] as a relatively low-powered, open-nesting bee, in contrast to the more highly powered cavity nesters, Apis cerana and Apis mellifera.

Figure 8 Apis laboriosa congregation overhang in Bhutanxiv
Two other follow up studies round off Underwood’s findings. Roubic and coworkersxv report a nesting range in Nepal of 2500-3000 m where bees forage up as high as 4100 m. They further note that Apis laboriosa build nests under rock ledges in deep, vertical river valleys, likely to avoid predation by bears noting nests constructed at lower elevations may well be occupied year round.
Woyke, Wilde and Wildexvi made a followup study in the Himalayan low altitude ‘warm zone’ in the winter of 1999. Referencing Underwood they noted that:
…dropping temperatures make even the lower altitude cliff sites inhospitable and colonies must leave the cliffs. They survive December and January in the forest in energy-efficient, comb-less clusters near the ground.
The Woyke group study was confined to the altitudinal zone between 1250 and 1500 m identifying sites with multiple (6-53) nests. They observed that some of the colonies were active, observed bees collecting pollen and water and found that comb collected by honey hunters had brood in all stages. Perhaps this confirms Roubic’s findings of year round occupation of some lower elevation nests. From their limited time observations, the Woyke team concluded that:
…colonies were not preparing themselves for abandoning the combs and for migration. Because colonies migrate when all or almost all brood emerges, those colonies would not migrate at least within three weeks [sic that] is till 10th of January. However, the amount of brood present and the young development stages, suggests that the colonies would occupy their combs for [a] longer period.
Clearly the migratory pattern of this honey bee is complex and contingent upon local microclimatic conditions and availability of forage. Woyke et al surmised:
…colonies from the cool areas above 2000 m migrate for winter into the lower warm areas, where they pass some time in comb-less clusters. However, established colonies in the warm areas, below 1500 m do not abandon their nest in winter, but remain on their combs rearing brood during that season.
Since they found abandoned dark comb at sites in their ‘warm’ observation area they postulated that, in summer, some or all colonies may abandon their combs in the ‘warm zone’ and migrate to higher altitudes.
The studies may also be confounded by the extensive traditional honey hunting by Himalayan communities, now exacerbated by ecotourism, where there appears to be a decline in colony occupation of overhang congregation sheltersxvii.
A fallout of this trend would appear to be a declining pollination service to orchards. Batraxviii further notes that colonies of the Giant Mountain Honey Bee in northern India may migrate in response to predation by the hornet, Vespa mandarinia.
The Giant Sulawesi Giant Honey Bee Apis dorsata binghami
All the forms resemble,
Yet none is the same as another;
Thus the whole of the throng
Points at a deep hidden law.
Johann Wolfgang von Goethe (1749-1832)xix
The Giant Sulawesi Honey Bee (Apis dorsata binghami) differs from its Asian mainland cousin, Apis dorsata (Apis dorsata dorsata), in having a longer tongue and longer wingsxx but whether it can be classified as a separate species is yet to be resolvedxxi.
Starr, Schmidt and Schmidtxxii review the status of the Giant Sulawesi Honey Bee (Apis dorsata binghami) in the regional context of both the Giant Philippine Honey Bee (Apis breviligula) and the common Giant Honey Bee (Apis dorsata dorsata). The debate on its position harks back to 1953 when Maa delineated the giant honey bees as three separate speciesxxiii. Here it is worth noting that the Giant Mountain Honey Bee, Apis laboriosa was not recognised until 1980xxiv. Contemporary taxonomists require more genetic informationxxv to ascertain whether binghami should be accorded separate species status.
The Giant Sulawesi Honey Bee is restricted naturally to the Sulawesi and surrounding islands: Nagir, Atmowidi. and Kahonoxxvi describe the characteristics of its nest, its nesting trees and its nesting behaviourin the Moros forests of the southern Sulawesi. Of the of one hundred and fifty two colonies they located (17 active nests, 85 abandoned combs) they found eleven nests at 0-11 meters, forty nests at 11-20 meters and fifty one nests at more than 21 meters above ground level and found them in thirty four different types of trees. Nests were all firmly attached to sound woody branches, all sloping from the main trunk by less than 60°. All nests were camouflaged by lianas and foliage.
Apart from a few images of nests and bees there appear to be no other reports of the natural history of this enigmatic honey bee.
The Giant Philippine Honey Bee Apis breviligula
This species, with a black rather than the normal yellow and black Apis dorsata abdomenxxvii, is confined to the main islands in the Philippines (Figure 9) long separated from the Asian mainland so evolved to become a new species. It does not occur on Palawan Island to the west that was once connected to Kalimantan and the Asian mainland. The giant honey bee in the Palawan Islands is the Asian mainland Apis dorsata dorsata.
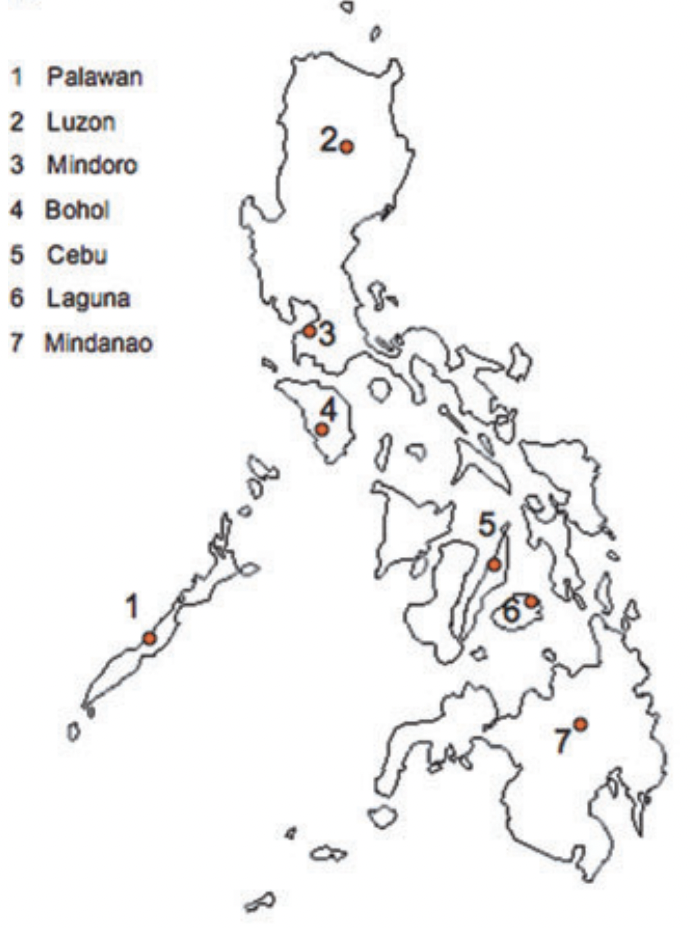
Figure 9 Distribution of Apis breviligula excluding the Palawan Islands
Source: Lo, Gloag, Anderson and Oldroyd (2010)
In an early description of the nest of the Giant Philippine Honey Bee, ‘Apis dorsata‘, now known as a distinct striped giant honey bee Apis breviligula, Roger (Doc) Morse gives a rare insight into its biologyxxviii. Of its temperament he notes:
Apis dorsata (sic breviligula) is the most ferocious of stinging insects. It is not uncommon for five to ten per cent of a nest population to attack an intruder within a few seconds of being disturbed. The workers will pursue a disturbing man or animal for long distances; the bees will pursue enemies into shaded areas to a greater extent than other Apis.
Nest populations varied from a low of 1000 to a high estimated at 70,000 in the thirty nests examined. Drone populations approximated those in Apis mellifera colonies.
…Between 80 and 95 per cent of the population may be used, depending upon weather conditions, to build a curtain of bees, several bees thick, around the comb. There is a bee space, or working space, between the curtain of bees and the comb. The bees in the curtain are inactive, hanging head upwards. When a colony swarms the curtain may be only one bee thick over the brood, but queen cells which are left behind are well protected with a heavy curtain of bees.
Further notes signal that the giant honey bee performs its dance on the bee curtain though in a restricted area. From his study Morse concluded that aspects of honey bee biology, such as nest temperature control and the concomitant well-defined brood rearing cycle, would have evolved early in the development of the genus. Nevertheless there are many subtle differences in the likes of swarming behaviour that set the giant honey bees apart from those of other honey beesxxix. Giant honey bees not only swarm to form new colonies nearby, they also abscond once the colony becomes weakened and they also migrate, typically twice per year – once to a distant locale when floral resources decline, then returning to either the swarmed location or to the parent hive vicinity – when the season returns to a honey gathering mecca.
Future of giant honey bees
Survival and spread
Before we leave the giant honey bees, letting them live their own lives, we might reflect and be concerned about their ultimate survival. They are extremely prone to habitat destruction (e.g. deforestation), honey hunting and shifts in climate. They also perform an essential function in pollinating tropical flora, crops and orchards. Their decline would see both the bees and the communities dependent of their ecosystem service as major losers.
It would however be naïve to think giant honey bees might be welcome elsewhere. Their spread – for example to the Near East, to the central Americas or to our region – presents a very real existential threat to tropical ecosystems and may cause panic – they are much more a risk to people than common or garden bees – in the general community. Further their potential to host Tropilaelaps (tropi) mites that would spread to and threaten our honey bee and regular sideline industry should not be underestimated. Tropilaelaps mercedesae – hosted by Apis dorsata – has gradually spread from the tropics and wreaked havoc in honey bee (Apis mellifera and Apis cerana) apiaries in colder climes. Worryingly, Tropilaelaps have been found to both outcompete and displace Varroa in hives co-infected with both parasites.
Role in spread of tropi mites
The grooming behaviour of the giant honey bee(s), as well as their propensity to migrate – resulting in breaks in the breeding cycle – greatly reduces parasite impact. The most coherent accounts of Tropilaelaps mites, and their associations with honey bees and their impacts, is provided by Anderson and Morganxxx with a more detailed analysis of the ecology and life history of parasites provided by de Guzman et alxxxi.
There are four Tropilaelaps species (Tropilaelaps mercedesae and Tropilaelaps thaii) distinctly separated from Tropilaelaps clareae and Tropilaelaps koenigerumxxxii. How, where and when they evolved is only now being unravelled. However some recognition of the geographical variability of their distribution and their association with different giant honey bees will be key to understanding their expanding impact not only on the cultivated Asian and Western Honey Bees but also to all other honey bees.
de Guzman et alxxxiii note that until fairly recently the two most common species were not clearly delineated suggesting that previous studies on T. clareae, conducted in areas east of the Wallace line, including New Guinea, be attributed to T. mercedesae, whereas those west of the Wallace line be referred to T. clareae. They present the main threat to the global apiary industry.
The Tropilaelaps mercedesae mite with its indigenous host, A. dorsata, is widely distributed on the Asian mainland excluding the Palawan Islands in the Philippines. The propensity of Apis dorsata to migrate long distances and for colonies to aggregate in large numbers, up to 100 per tree, may account for this mite being so widely distributed. These mites now infect other species, Apis laboriosa, Apis cerana and introduced Apis mellifera across Asia.
The native host of the related Tropilaelaps thaii is the Giant Mountain Honey Bee Apis laboriosa from the Himalaya region.
Tropilaelaps clareae presents a similar threat. For example it infests Apis mellifera colonies across the Philippinesxxxiv (except on the Palawan Islands). Its sparse distribution has been attributed to the isolation of its adapted hosts, the Giant Sulawesi Honey Bee (Apis dorsata binghami) and the Giant Philippine Honey Bee (Apis breviligula) and to the fact that unlike Apis dorsata, Apis breviligula colonies do not aggregatexxxv.
Tropilaelaps koenigerum, related to Tropilaelaps clareae, is the smallest mite of this genus and is also a parasite of Apis dorsata in mainland Asia and Indonesia. Both Tropilaelaps koenigerum and T. thaii appear not to affect the western honey beexxxvi.
That Tropilaelaps may transform the existing beekeeping industry more radically than has Varroa should give us all considerable room to reflect.
Readings
iHepburn, R. and Radloff, S.E. (Eds) (2011). Honeybees of Asia. Berlin Springer-Verlag. doi:10.1007/978–3–642–16422–4
iiOldroyd, B.P. and Wongsiri S. (2006). Asian honey bees, 360pp. Cambridge: Harvard University Press.
iiiPaar, J., Oldroyd, B.P., Huettinger, E. and Kasterberger, G. (2004). Genetic structure of an Apis dorsata population: The significance of migration and colony aggregation. Journal of Heredity 95(2):119–126. doi:10.1093/jhered/esh026 https://sci-hub.mksa.top/10.1093/jhered/esh026
ivHepburn and Radloff (2011) loc. cit. Chapter 7, Absconding, migration and swarming, pp.133-158 and Chapter 3 Biogeography.
vWoyke, Wilde and Wilde (2012) loc.cit.
viRobinson, W.S. (2012). Migrating giant honey bees (Apis dorsata) congregate annually at stopover site in Thailand. Plos One 7(9): e44976. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3446981/
viiUnderwood, B.A. (1990). Seasonal nesting cycle and migration patterns of the Himalayan honeybee, Apis laboriosa. National Geographic Research 6:276–290. https://www.researchgate.net/publication/279756773_Seasonal_nesting_cycle_and_migration_patterns_of_the_Himalayan_honey_bee_Apis_laboriosa
Underwood, B.A. (1992). Impacts of human activities on the Himalayan honey bee, Apis laboriosa, Honey Bee Resources inVerna, L.R.(ed). Honeybees in Mountain Agriculture, Oxford & IBH, New Delhi, Chapter 4, pp.51-57. http://lib.icimod.org/record/25252/files/c_attachment_411_5173.pdf
viiiWoyke, J. and Wilde, M. (2003). Periodic mass flights of Apis laboriosa in Nepal. Apidologie 34:121–127. https://hal.archives-ouvertes.fr/hal-00891762/document doi:10.1051/apido:2003002
Woyke, J., Wilde, J. and Wilde, M. (2012). Swarming and migration of Apis dorsata and Apis laboriosa honey bees in India, Nepal and Bhutan. Journal of Apicultural Science 56(1):81-91. doi:10.2478/v10289-012-0009-7 https://sciendo.com/article/10.2478/v10289-012-0009-7 https://sciendo.com/downloadpdf/journals/jas/56/1/article-p81.xml https://www.degruyter.com/downloadpdf/j/jas.2012.56.issue-1/v10289-012-0009-7/v10289-012-0009-7.pdf
ixFor an informative narrative of cliff-side hunting, see the video documentary by Treza, R. (2013). Hallucinogen honey hunters: Hunting mad honey, YouTube, 26 September 2013, 26:39. https://youtu.be/Y_b2i_FvYPw
xTan, N.Q. and Ha, D.T. (2002). Socio-economic factors in traditional rafter beekeeping with Apis dorsata in Vietnam. Bee World 83(4):165-170. doi.org/10.1080/0005772X.2002.11099559 https://www.tandfonline.com/doi/abs/10.1080/0005772X.2002.11099559
xiKitnya, N., Prabhudev, M.V., Bhatta, C.P., Pham, T.H., Nidup, T., Megu, K., Chakravorty, J., Brockmann, A. and Otis, G.W. (2020). Geographical distribution of the giant honey bee Apis laboriosa Smith, 1871 (Hymenoptera, Apidae). ZooKeys 951:67-81. doi.org/10.3897/zookeys.951.49855 https://zookeys.pensoft.net/article/49855/
xiiWoyke, J., Wilde, J. and Wilde, M. (2001). A scientific note on Apis laboriosa winter nesting and brood rearing in the warm zone of Himalayas. Apidologie 32(6):601–602. doi:10.1051/apido:2001104 https://www.academia.edu/3397843/A_scientific_note_on_Apis_laboriosa_winter_nesting_and_brood_rearing_in_the_warm_zone_of_Himalayas
https://hal.archives-ouvertes.fr/hal-00891911/document
doi:10.1051/apido:2001104
xiiiUnderwood, B.A. (1990). Seasonal nesting cycle and migration patterns of the Himalayan honey bee Apis laboriosa. National Geographic Research 6(3):276–290. https://eurekamag.com/research/007/773/007773060.php
https://www.researchgate.net/publication/279756773_Seasonal_nesting_cycle_and_migration_patterns_of_the_Him alayan_honey_bee_Apis_laboriosa
Underwood, B.A. (1990). The behaviour and energetics of high-altitude survival by the Himalayan honeybee, Apis laboriosa. PhD thesis, Cornell University, Ithaca, USA. 144pp. https://www.cabdirect.org/cabdirect/abstract/19910230590
xivGregory, M., and Jack, C. (February 2022). Himalayan giant honey bee, cliff honey bee Apis laboriosa Smith (Insecta: Hymenoptera: Apidae). University of Florida. https://edis.ifas.ufl.edu/publication/IN1348 doi.org/10.32473/edis-IN1348-2022
xvRoubik, D.W., Sakagami, S.F. and Kudo, I. (1985). A note on distribution and nesting of the Himalayan honey bee Apis laboriosa Smith (Hymenoptera: Apidae). Journal of the Kansas Entomological Society 58(4):746-749. https://www.jstor.org/stable/25084723
xviWoyke, J., Wilde, J. and Wilde, M. (2001). A scientific note on Apis laboriosa winter nesting and brood rearing in the warm zone of Himalayas. Apidologie 32(6):601–602. doi:10.1051/apido:2001104 https://www.academia.edu/3397843/A_scientific_note_on_Apis_laboriosa_winter_nesting_and_brood_rearing_in_the_warm_zone_of_Himalayas
https://hal.archives-ouvertes.fr/hal-00891911/document
doi:10.1051/apido:2001104
xviiJoshi, S.R, Ahmad, F. and Gurung, M.B. (2004). Status of Apis laboriosa populations in Kaski district, western Nepal. Journal of Apicultural Research 43(4):176–180. https://www.researchgate.net/publication/292620321_Status_of_Apis_laboriosa_populations_in_Kaski_district_western_Nepal
Ahmad, F., Gurung, M.B. and Joshi, S.R. (2003). The Himalayan cliff bee Apis laboriosa & honey hunters of Kaski, 71pp. https://shop.beesfordevelopment.org/products/the-himalayan-cliff-bee-apis-laboriosa-honey-hunters-of-kaski downloaded 20 May 2022 by searching at The Himalayan Cliff Bee Apis laboriosa – ICIMODhttps://lib.icimod.org › files › attachment_124PDF
xviiiBatra, S.W.T. (1996). Biology of Apis laboriosa Smith, a pollinator of apples at high altitude in the Great Himalaya Range of Garhwal, India, (Hymenoptera: Apidae). Journal of the Kansas Entomological Society 69(2):177-181. https://www.jstor.org/stable/25085665
xixLewes, G.H. (before 1885). The life and works of Goethe, Volume 1, p.347. Second edition, London:Smith, Elder, and Co,.,65, Cornhill, 1864. https://books.google.com.au/books?id=9H8oAAAAMAAJ&pg=PA347&lpg=PA347&dq=goethe+Points+at+a+deep+hidden+law&source=bl&ots=4nymCZZg7J&sig=ACfU3U1QeXtcCDvLJ8yrvAP5p6_ZIXQFcg&hl=en&sa=X&ved=2ahUKEwiI9-20peb3AhUnUGwGHX6wBOMQ6AF6BAgsEAM#v=onepage&q=goethe%20Points%20at%20a%20deep%20hidden%20law&f=false
xxUniversity of Florida. (downloaded 17 May 2022). Featured creatures: Apis dorsata Fabricius (Insecta: Hymenoptera: Apidae). https://entnemdept.ufl.edu/creatures/MISC/BEES/Apis_dorsata.htm
xxiLo, N., Gloag, R.S., Anderson, D.L. and Oldroyd, B.P. (2010). A molecular phylogeny of the genus Apis suggests that the giant honey bee of the Philippines, A. breviligula Maa, and the Plains Honey Bee of southern India, A. indica Fabricius, are valid species. Systematic Entomology 35(2):226–233. The Royal Entomological Society. doi:10.1111/j.1365-3113.2009.00504.x
xxiiStarr, C.K., Schmidt, P.J. and Schmidt, J.O. (1987). Nest-site preferences of the giant honey bee, Apis dorsata (Hymenoptera: Apidae), in Borneo. Pan-Pacific Entomologist 63(1):37-42. http://www.ckstarr.net/cks/1987-MEGAPIS.pdf
xxiiiMaa, T.C. (1953). An inquiry into the systematics of the tribus Apidini or honeybees (Hym.). Treubia 21(3):525-640. https://e-journal.biologi.lipi.go.id/index.php/treubia/article/view/2669/2299
xxivSakagami, S.F., Matsumura, T. and Ito, K. (1980). Apis laboriosa in Himalaya, the little known world largest honeybee (Hymenoptera, Apidae). Insecta Matsumurana 19:47–78. https://eprints.lib.hokudai.ac.jp/dspace/bitstream/2115/9801/1/19_p47-77.pdf
Darlington, P. Jr. (1957). Zoogeography: The geographical distribution of animals, 694pp., John Wiley, New York. Available at the Australian National Library: https://catalogue.nla.gov.au/Record/2516011
xxvSee for example Lo, Gloag, Anderson and Oldroyd (2010) loc.cit.
xxviNagir, M.T., Atmowidi, T. and Kahono, S. (2016). The distribution and nest-site preference of Apis dorsata binghami at Maros Forest, South Sulawesi, Indonesia. Journal of Insect Biodiversity 4(23):1-4. doi:10.12976/jib/2016.4.23 https://www.researchgate.net/publication/311972586_The_distribution_and_nest-site_preference_of_Apis_dorsata_binghami_at_Maros_Forest_South_Sulawesi_Indonesia
xxviiLo, Gloag, Anderson and Oldroyd (2010) loc. cit.
xxviiiMorse, R.A. (1969). The biology of Apis dorsata in the Philippines. International Union for the Study of Social Insects Proceedings pp.185-187. https://cataglyphis.fr/Actes-SF-UIEIS/IUSSI-Bern-1969/IUSSI-Bern-1969-Morse.pdf
Morse, R.A. and Laigo, F.M. (1969). Apis dorsata in the Philippines (including an annotated bibliography) Laguna, Monograph of Philippine Association of Entomologists 1:1-96. https://www.worldcat.org/title/apis-dorsata-in-the-philippines-including-an-annotated-bibliography/oclc/63374047#similar https://www.amazon.com/Apis-dorsata-Philippines-bibliography-Entomologists/dp/B0007JS7TK
Additional notes and correspondence are posited with the The National Library of Wales [Llyfrgell Genedlaethol Cymru] as GB 0210 BEEIBRA International Bee Research Association (IBRA) Records:Reference code: CP1/29. Vtls006326885 and physically located at ARCH/MSS (GB0210) in a folder entitled Morse R.A. and Laigo, F.M. (1968). The biology of Apis dorsata parts iii, v, vii and ix,] https://archiveshub.jisc.ac.uk/data/gb210-beeibra/cp1/29 https://archiveshub.jisc.ac.uk/search/archives/cd8e66d5-b792-306a-9073-7d6b54d3b8ce?component=c82b8c51-4ac6-38cc-b020-809d542ff657
xxixWoyke, Wilde, and Wilde (2012) loc. cit.
xxxAnderson, D.L. and Morgan, M.J. (2007). Genetic and morphological variation of bee-parasitic Tropilaelaps mites (Acari: Laelapidae): New and re-defined species. Experimental and Applied Acarology 43(1):1–24. doi:10.1007/s10493-007-9103-0
xxxide Guzman, L.I., Williams, G.R., Khongphinitbunjong, K. and Chantawannakul, P. (2017). Ecology, life history, and management of Tropilaelaps mites. Journal of Economic Entomology 110(2):319–332. doi.org/10.1093/jee/tow304
xxxiiAnderson and Morgan (2007) loc. cit.
xxxiiide Guzman, Williams, Khongphinitbunjong, and Chantawannakul. (2017) loc. cit.
xxxivLaigo, F.M. and Morse, R.A. (1968). The mite Tropilaelaps clareae in Apis dorsata colonies in the Philippines. Bee World 49(3):116-118. doi.org/10.1080/0005772X.1968.11097211
xxxvMorse, R.A. and Laigo, F.M. (1969). Apis dorsata in the Philippines (including an annotated bibliography) Laguna, Monograph of Philippine Association of Entomologists 1:1-96. https://www.worldcat.org/title/apis-dorsata-in-the-philippines-including-an-annotated-bibliography/oclc/63374047#similar https://www.amazon.com/Apis-dorsata-Philippines-bibliography-Entomologists/dp/B0007JS7TK
xxxviFranco, S. (2018). OIE Terrestrial Manual, Chapter 3.2.6. Infestation of honey bees with Tropilaelaps spp. pp.765-776. OIE World Organisation for Animal Health. https://www.oie.int/fileadmin/Home/fr/Health_standards/tahm/3.02.06_TROPILAELAPS.pdf
