Alan Wade
What do we really know about the social lives of the corbiculate bees we keep, the honey, the stingless and the bumble bees, assuming we are not also keepers of orchid bees?i Quite a lot actually especially if you delve deep into the voluminous scientific literature describing their biology and their origins. Sometimes lacking are comparisons between each of the tribe life systems. What might we learn to help us better understand bees or to keep them a whole lot better?
There are seven bee families, three sub families of which contain taxa that display a wide range of social behaviours. The advanced eusocial taxa are found wholly within just two families, the Halictidae (one subfamily) and the Apidae (two subfamilies). Amongst these are the perspiration attractant sweat bees (in the sub family Halictinae), the large chippy chip carpenter bees (sub family Xylocopinae) and the familiar honey storing corbiculate bees (sub family Apinae) (Figure 4.1). Our focus is now on the apine corbiculate bees.
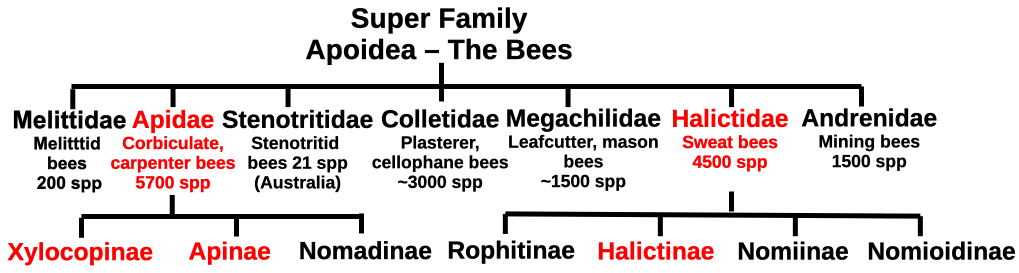
Figure 4.1 The bees showing families and sub families with eusocial species.
Eusocial behaviour – colonies with a helper worker class – evolved independently in each of these sub families, the Xylocopinae, the Apinae and the Halictinae. Not only are distinctly different expressions of eusociality found in each tribe of these subfamilies but also between genera and even within species.
Of the four living tribes that comprise the Apinae (the corbiculates), three contain the bulk of all eusocial bee species, the Apini (the honey bees), the Meliponini (the stingless bees) and the Bombini (the bumble bees). The fourth tribe, the Euglossini or orchid bees, contain a mixture of communal taxa, colonies with multiple queens that are emphatically neither solitary nor eusocial, and many solitary species. The progenitor of all the tribes was a common facultatively eusocial bee from which the eusocial tribes evolved and from which solitary behaviour in the Euglossini was reinvented.
Corbiculate origins
Overall there are about 15,000 species of bees belonging to the super family Apoidae. The vast majority are solitary insects, many also found in the giant family Apidae with a total of around 5700 species. These bees can all be traced back to their coevolution with the flowering plants in the early Cretaceous 113-132 million years ago (mya)ii when the original primitive crown beesiii separated from the aculeate stinging wasps.
Sadd and coworkersiv, summarising the findings of Cardinal and Danforth,v state that:
The four tribes of corbiculate bees, Apini, Meliponini, Bombini, and Euglossini, are thought to have shared a primitively eusocial ancestor. Subsequently, the Meliponini and Apini evolved advanced eusociality independently, while the predominantly solitary behavior of the Euglossini was secondarily derived. Although rare overall, advanced eusociality has arisen twice in this group, once following the split of honeybee and bumblebee lineages (c. 77–95 mya), and once following the split of stingless bee and bumblebee lineages (c. 66–82 mya).
Cardinal and Danforthvi postulate that primitive eusocial behaviour originated just once in the mid Cretaceous 87-95 mya. From there the corbiculate tribes diverged, a few becoming extinct. Three followed separate pathways to full obligate eusociality while the remaining orchid bee tribe reinvented a solitary, sometimes communal, lifestyle. We first discover that the permanent obligate eusocial behaviour found in the honey bees is expressed quite differently in the stingless bees. They both form permanent nests but their strategies for survival and reproduction evolved entirely independently. Eusocial expression is yet different again amongst the bumble bees where the social fabric completely breaks down every autumn. Colonies are only reestablished after overwintering foundress queens start new colonies each spring. The bumble bee life cycle thus alternates between solitary and obligate social behaviour. Eusociality is a temporary arrangement and sets bumble bees apart from both the honey bees and stingless bees.
While no single ant or social corbiculate bee or wasp can adopt a ‘singles lifestyle’, eusociality continues to find new expression. This is clearly evident amongst the well studied honey bees. For example Oldroyd and Prattvii posit that the Black Dwarf Honey Bee Apis andreniformis separated from the Red Dwarf Honey Bee Apis florea as recently as 10,000 years ago, that is since the end of the last ice age (11,700 ybp during the present Holocene). The very close affinity of the two dwarf bee species is evidenced by their sharing many common attributes: for example queens can be swapped between colonies of the two species and their natural domains overlap. However their behaviours also differ: Apis andreniformis is far more likely to abscond than Apis florea. It nests away from human settlement and its comb attachment is quite different (Figure 4.2).
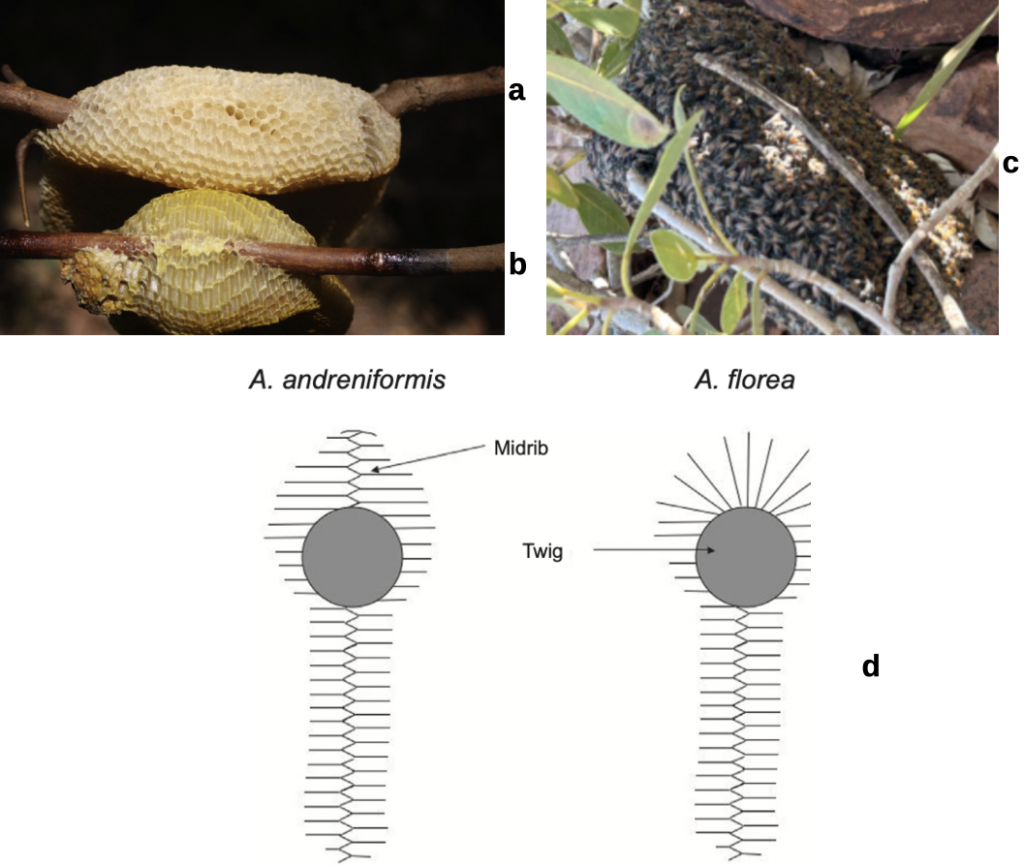
Figure 4.2 Dwarf honey bee comb attachment:
(a) Apis florea comb;
(b) Apis andreniformis comb; and
(c) Apis florea colony, Karratha, Western Australia; and
(d) nest discrimination schematic.
In like manner the giant honey bees, Apis breviligula (the Phillipines Giant Honey Bee), Apis dorsata binghami (the Sulawesi Giant Honey Bee)viii and the mountain adapted Giant Cliff Honey Bee (Apis laboriosa) have all drifted from the basal and widespread Apis dorsata (Apis dorsata dorsata) as a result of geographic island drift during the Pleistocene,ix that is since the early ice ages 2.58 mya.
Corbiculate eusociality exemplified
But where overall do these corbiculate bee origins and evolved eusociality now leave each of the tribes? How might we best describe their disparate behaviours?
With good evidence that they had a single common primitively eusocial ancestor, we now also know from the hallictid sweat bees (the Halictini and Augochlorini tribes) and apid carpenter bees (the Allodapini and Ceratinini tribes) what primitive forms of corbiculate eusociality may have actually looked like. Corbiculate obligate eusocial traits evolved over a longer timeframe (Figure 4.3).
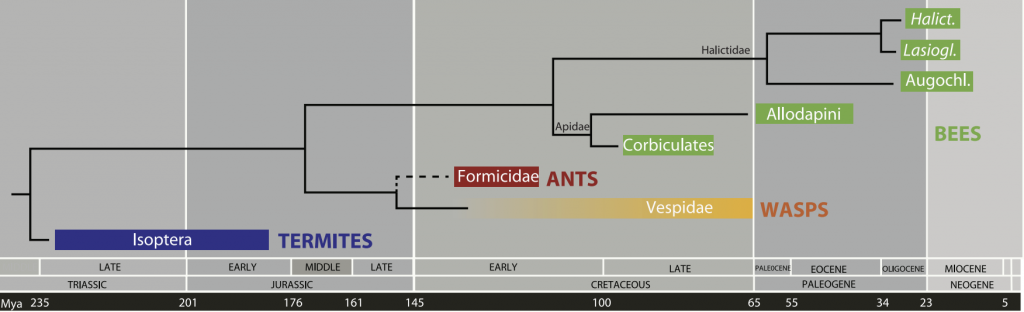
Figure 4.3 Estimated times of emergence of the major eusocial insect clades.x
Note: The carpenter bees (Allodapinae: Allodapini and Ceratinini) emerged much later than the corbiculates (Apinae: Apini, Meliponini, Bombini and Euglossini) and later still in the sweat bees (Halictinae: Halictini and Augochlorini).
Porto and Almeida outline corbiculates phylogeny in terms of their morphology.xi Their examination of wing venation and other body characteristics favoured an Apini + Meliponini association, while they observed that molecular characters more closely aligned Meliponini with Bombini. From an exhaustive analysis of morphological traits (body shape and patterning features) they concluded that Apini was a sister to Meliponini, together a sister to Bombini, an intuitive response we might all have made from the fact that honey and stingless bees are permanently eusocial while bumble bees are more aligned behaviourally with the facultatively eusocial bees found amongst the sweat and carpenter bees. They conclude:
One interpretation is that the fixed-caste eusociality, also referred to as advanced [or obligate] eusociality, could have arisen independently in Apini and Meliponini or alternatively it could be thought of as an ancestral condition for the clade comprising Apini, Bombini, and Meliponini, but later modified in bumble bees.
From the review of the Halictinae sweat bee sub family (Part II) and the Xylocopinae carpenter bee sub family (Part III), we can now see more clearly how the eusocial corbiculate bees, aculeate waspsxii and the less related ants evolved (Figure 4.4).
Cardinal and Danforth outline the independent evolution of permanent eusociality in the honey and stingless bees:
From this primitively eusocial ancestor, stingless bees and honey bees independently evolved advanced eusocial behavior. Our life-history traits analyses indicate that honey bees and stingless bees inherited castes with division of labor and colonies containing adults of two generations (characteristics of primitively eusocial colonies) from the common ancestor of corbiculates as a whole. However, they appear to have convergently evolved morphological differentiation between reproductive and worker castes and swarming behavior (characteristics of highly eusocial colonies). The evolution of advanced eusociality has only occurred a handful of times, and thus it is particularly noteworthy that it has evolved twice within this one, relatively small, clade of bees.
While remarkable, a hypothesis of dual origins of advanced eusociality is congruent with early studies on corbiculate morphology and social behavior. Though Meliponini and Apini both have evolved elaborate social behavior, they differ substantially in the details of their social biology.
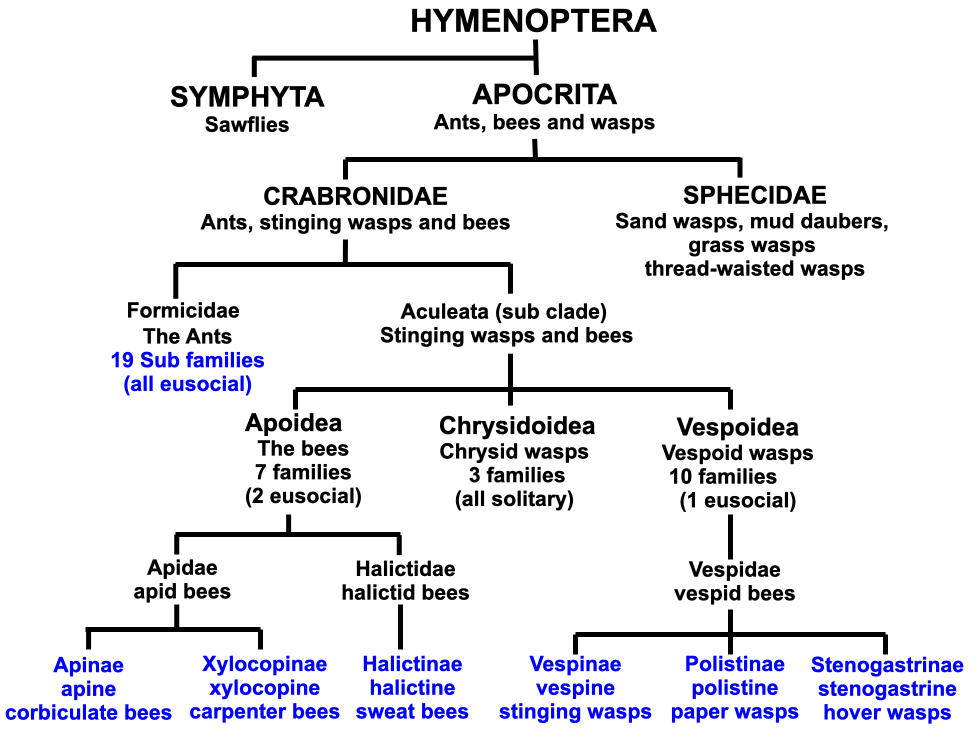
Figure 4.4 Relationships between subfamilies containing eusocial ants, bees and wasps.
Broad attributes of the Apinae corbiculate tribes are outlined in Table 4.1.
| Apinae tribe | Social level | Colony reproduction | Number of species | Larval provisioning | Queen replacement [mating] |
| Apini (honey bees) tropical and temperate [monogynous/polyandrous] | Obligate permanent eusocial – queen dominant | Swarming (precipitate fission): Old and secondary virgin queen migration with swarms | 12 [single genus Apis] | Progressive larval provisioning with differential worker and queen diets | Supersedure, swarm and emergency queen replacement |
| Meliponini (stingless bees) neotropical and warm temperate | Obligate permanent eusocial – queen dominant may harbour virgin queens | Swarming (assisted fission): Virgin queen migration once new colony established | ~ 500 (some cryptic) [~57 genera, e.g. Australia Tetragonula, Austroplebeia] | Mass provisioning of cell batches where diet is not always the determining factor in caste delineation | Regular replacement by reserve virgin queens [monogynous*/ monandrous or very occasionally polyandrous**] |
| Bombini (bumble bees) temperate | Obligate temporary eusocial – queens and workers vie for dominance | Foundress queen nest establishment | ~ 250 (some parasitic) [single genus Bombus] | Batch provisioning but progressive | Not replaced. May be superseded by invading queen in some spp. Workers assume dominance in queenless colonies. [monogynous/monandrous and polyandrous] |
| Euglossini (orchid bees) neotropical | Commonly solitary but also communal (multi queened) | Solitary brood raising sometimes in communal setting | ~200 [5 genera, Aglae and Exaerete kleptoparasites; Euglossa, Eulaema, Eufriesea] | Sequential cell provisioning. | Generational turnover [Solitary/monandrous] |
| Electrobombini (extinct)*** | Bombini affinity | Electrobombus | Close to bumble bees | ||
| Electrotrapini (extinct)*** | Between Bombini and Apini | Electrapis | Bumble bee-ike | ||
| Melikertini (extinct)*** | Meliponini affinity | Mellissites Melikertes Roussyana Succinnapis | Stingless bee-like |
Table 4.1 Common traits delineating the corbiculate tribes.
*Two species known to be multi queened.
**Stingless bees are generally considered to be monandrous (i.e. singly mated)xiii but a study of the genetics of two species, Melipona beecheii and Scaptotrigona postica suggests that polyandry also exists.xiv Thus females in a stingless bee colony are almost invariably more closely related to one other than they are in honey bee colonies where there are many super sister worker groups in the one colony.
***Extinct tribes are described by Engle.xv
While the corbiculate bee tribes are emphatically distinguishable from one another, we might also ask ourselves in what specific ways do they differ? As superorganisms they certainly rival vertebrate animals in terms of their biological organisation and in many ways far surpass the complexity of social arrangements of herd animals such as ourselves.
Some of the mystique surrounding the corbiculates is buried in the variety of their social expression. Cardinal and Danforth distil this succinctly in stating that:xvi
The advanced eusocial Apini and Meliponini have morphologically distinct queens and workers with new nests founded by swarms, whereas the primitively eusocial Bombini have queens and workers that differ only in size, with new nests established by a single foundress. Non-parasitic orchid bees are usually referred to as being solitary or communal, but hints of more advanced forms of social behavior, including overlap of generations and cooperative brood care, have been reported in some taxa.
They report further on tribal differences based on behavioural and nesting traits, a result of their having evolved independently. Overarching differences are most clearly evident in nest architecture, in nest building materials, in population dynamics, in brood nest functioning and in the behavioural traits exhibited by each of the different bee castes. Engels and Imperatriz-Fonsecaxvii note that while corbiculate evolution has never resulted in workers becoming an entirely sterile worker caste, this is a condition they argue is theoretically possible. Instead the interplay between female queen and worker castes is a not as clear cut as is sometimes made out. Both honey and stingless bee workers can lay drone eggs, a trait heavily suppressed in queenright colonies. However amongst bumble bees, where queens and workers differ in size only, both queenright and worker-led colonies can thrive. Indeed amongst bumble bees colonies there is always a tussle for colony dominance between the queen and workers. Queen loss results in more dominant colony workers taking control of the nest, albeit without the potential to produce female workers and queens. The queenless colony stabilises and produces large numbers of drones, queenright colonies producing both queens and drones late in the season. Together they enhance the reproductive success of bumble bees measured in the number of mated foundress queens that go into diapause.
Corbiculate tribes in profile
Drawing on morphological and molecular data, Grüter distils a coherent model for the evolutionary relationship between the corbiculate tribes (Figure 4.5) explaining that:
More recent analyses provide further support for a dual origin of higher eusociality in corbiculate bees and a sister group relationship between stingless bees and bumble bees. According to the currently best supported scenario (Figure 4.5), eusociality evolved once in the common ancestor of the Apini, the Bombini and the Meliponini.xviii This suggests that the ancestors of present-day honey bees and stingless bees independently evolved a highly eusocial lifestyle, i.e. distinct morphological female castes, perennial colonies and extensive food sharing. Two separate origins of higher eusociality would help to explain why honey bees and stingless bees have found markedly different solutions to the problem of colony reproduction, brood rearing, colony defence or foraging communication.
As a sub family, corbiculate bees are principally cavity dwelling, many bumble and stingless bees establishing colonies in both underground and protected above ground nests. Others, mainly the intermediate-sized Western and Asian honey bees, as well as many stingless bees, nest in tree hollows, rock crevices or man made containers sheltered from the elements whereas only the primitive dwarf and giant honey bees always nest in the open.
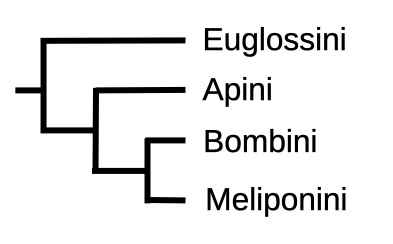
Figure 4.5 Grüter’s preferred model of corbiculate tribe phylogeny.
Corbiculate bees are almost ubiquitous. After ants, they are amongst the most common of all insects, though the Pacific and New Zealand are devoid of native corbiculate taxa and Australia has just eight species of stingless bee.
Let’s compare the tribes to see what attributes they share and what sets them apart from one other.
Honey bees and stingless bees compared
In a broad ranging overview of the Apini (honey bee) and Meliponini (stingless) bee tribes, Engles and Imperatriz-Fonseca, identify a key commonality and difference:xix
…only in honey bees and stingless bees is the queen incapable of founding a colony alone and of rearing the brood on her own;
and conversely:
…the typical queen and worker characteristics are different from one another not just with regard to the reproductive organs but include many other morphological, physiological, and behavioral differences as well. These striking caste distinctions are the result of postembryonic divergences in development which depend on ecological conditions, mainly on the external factor of larval nutrition.
The truly defining characteristics of honey and stingless bees are that their colonies are perennial and that they are always poised to raise a new generations of workers, drones and queens, unseasonal and poor conditions excepted. Engles and Imperatriz-Fonseca state this in a nuanced manner:
In the highly eusocial bees, worker brood is produced more or less constantly. The only exceptions are during hibernation and during swarming in honey bees and during seasonal breaks caused by drought, heavy rainfalls or low temperatures in stingless bees.
This bee replacement luxury is not afforded to solitary or to most other social bees though there are exceptions. An extreme example of the regenerative capacity of the permanently eusocial colony is found in the Italian honey bee, Apis mellifera ligustica. Möbus records periodic development of brood in honey bee hives, likely the hardy European honey bee Apis mellifera mellifera, that he examined in the dead of winter.xx Such proclivity to reproduce is amply demonstrated by a fellow beekeeper’s winter 2023 observations of a hive of Italian honey bees housed at Questacon, Australia’s National Science and Technology Centre (Figure 4.6). Canberra has a cold frosty temperate climate where most colonies become broodless in winter but where prosperous colonies very occasionally maintain drone populations year round. Brood temperature regulation appears to be a primordial trait amongst honey bees, being also recorded for Apis ceranaxxi and Apis dorsata.xxii
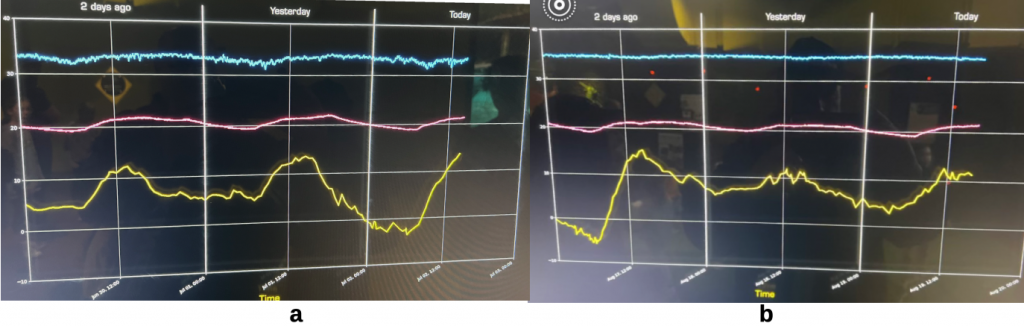
Figure 4.6 Italian honey bees raising brood at 35 0C throughout winter 2023:
yellow = ambient air temperature; pink = Science Centre air temperature; blue = top bar gap above brood nest:
(a) nest temperature plot late June 2023; and
(b) nest temperature plot late August 2023.
Note: The broodless winter cluster temperature in Apis mellifera is 18-20 0C.
Stingless bee strategy for brood nest temperature regulation is somewhat different to that adopted by honey bees. Pan tropical bees stingless bees have not evolved to cluster and shiver to keep brood warm. Instead they maintain nest homeostasis by a combination of brood nest insulation afforded by cavity wall material, a trait they share with honey bees, by surrounding their nest with a shielding involucrum and by fanning brood under overly hot conditions. Nevertheless the extent of brood nest temperature regulation is extremely varied across the stingless bee tribe mainly modulated by a wider spectrum of nesting types (e.g. subterranean nesting of some species). Overall stingless bees are raised on a much longer timeframe than are honey bees, according to Grüter ~33-55 days for stingless worker bees and 18-21 days (as we shall see in Part V) for Apis species bees, with implications for their population dynamic. Both honey and stingless bees nevertheless down regulate their populations in times of dearth adapting to seasonal and climatic resource availability conditions.
One other key difference between the tribes lies in their species diversity. While there are few honey bee species there are a plethora of genera and species of stingless bees.
Arias and Sheppardxxiii indicate that there are ten honey bee species: the giant bees (Apis dorsata, Apis breviligula and Apis laboriosa); the dwarf bees (Apis andreniformis and Apis florea); and the cavity-nesting bees (Apis mellifera, Apis cerana, Apis koschevnikovi, Apis nuluensis and Apis nigrocincta). With the recent addition of the Sulawesi Giant Honey Bee (Apis binghami) – disputably a separate species – and the Indian Plains Honey Bee (Apis indica), we might conclude that there are twelve separate species.
Stingless beesxxiv have been classified into fifty seven genera and a total of at least 550 mainly pan tropical species (Table 4.2).
| Region | Number of genera | Key genera | Number of species |
| Neotropics (Central America) | 30 | Melipona, Plebeia, Partamona, Trigonisca, Paratrigona, Scaptotrigona, Geotrigona, Lestrimelitta | ~ 426 |
| Afrotropics | 10 | Liotrigona, Axestotrigona, Hypotrigona | ~ 36 |
| Indo-Malay/Australasia | 17 | Tetragonula, Lepidotrigona, Austroplebeia | ~ 90 |
| Total | ~ 57 | ~550 |
Table 4.2 Global diversity and distribution of stingless bees.
Source: Modified from chart presented by Christoph Grüter.xxv
Melo describes the stingless bees as having:xxvi
…very high species diversity with extreme variation in body size, large, complex societies with strong queen worker dimorphism, elaborate nest structures, diverse foraging behavior and diet, repeated evolution of obligatory cleptobiotic behavior, and a vestigial venom apparatus.
He divides Meliponini species into the rich New World clade of central and South America and the sparse Indo-Asian group with convergently and superficially similar morphologies. He acknowledges the studies of Antonelli and coworkersxxvii in attributing the speciose South American stingless bee fauna to the Amazonian habitat diversity that has existed for the past 66 million years.
The natural ranges of the honey and stingless bees are depicted in Figure 4.7.
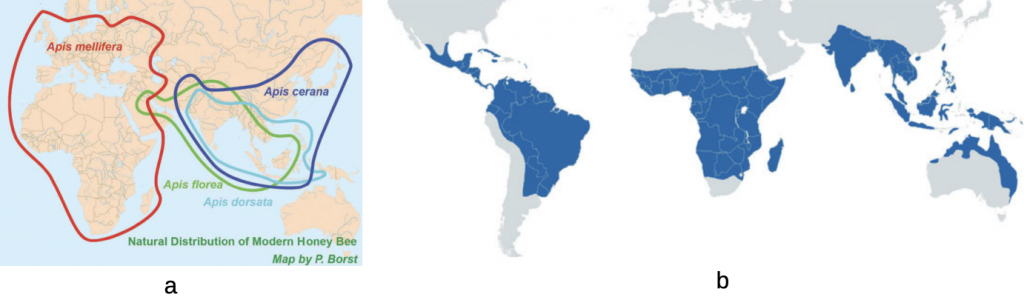
Figure 4.7 Natural distribution of advanced eusocial bees:xxviii
(a) clades of Afro-European and Indo-Asian honey bees; and
(b) pan tropical South American, African and Indo-Asian stingless bees.
The permanently eusocial honey and stingless bee tribes are of pan tropical origin whereas the bumble bees are largely temperate and are intermittently eusocial: the latter start up each year with foundress queens and are not adapted to support populations over extended times of dearth.
Nest thermoregulation and a unique capacity to forage efficiently to accumulate large stores has enabled cavity dwelling honey bees to radiate to high latitudes. Of course honey bees, as well as bumble bees and some pollinator solitary species such as megachilids, now have a much greater distribution due to human agency. We have spread them (and their mites and pathogens) far and wide.
A further defining characteristic of the corbiculate bees is their dependence on natural tree cavities or rock crevices, abandoned rodent and bird nests and more for shelter. Many corbiculates modify their chosen cavity by removing debris and making extensive use of natural materials such as plant resins to line their nests. Generalised representation of nests of the cavity dwelling honey bees are depicted by Tom Seeley and Roger Morsexxix and for stingless bees by Charles Michener (Figure 4.8).xxx Honey bees always nest above ground, either attached to small branches (dwarf honey bees), to tree branches, buildings or cliff overhangs (giant honey bees) or in tree hollows or rock crevices (cavity dwelling Asian and Western honey bees). Stingless bees have a diverse range of nesting preference. While many nest in tree hollows and rock cavities, some nest in the open or even underground, the pattern depending largely on the taxon. Bumble bees with typically smaller populations occupy natural below ground, e.g. abandoned rodent and vole, nests, or near above ground makeshift nests.
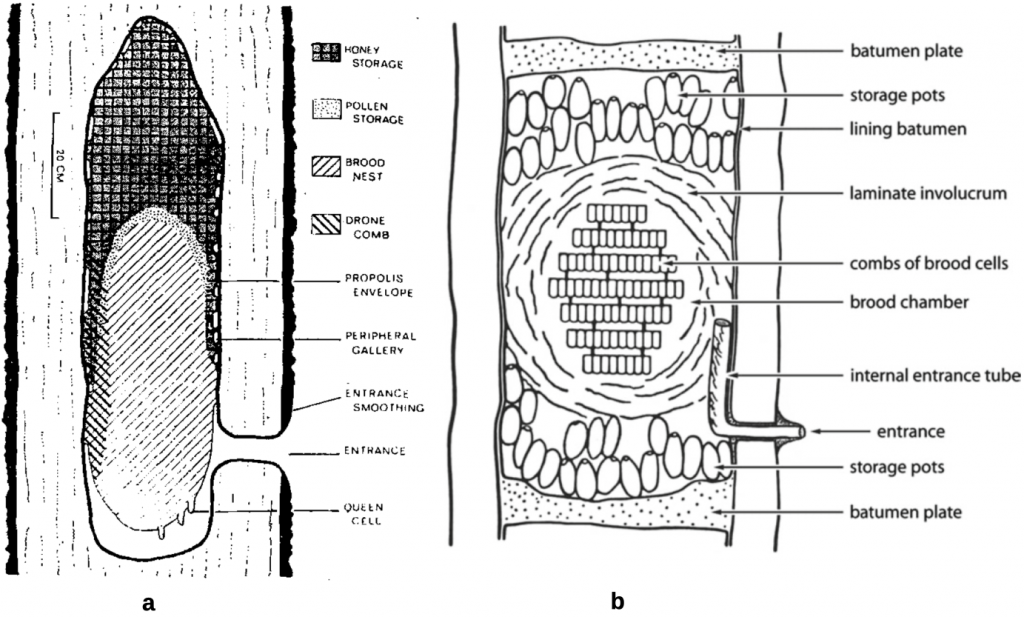
Figure 4.8 Generalised view of bee nests:
(a) the Apis mellifera honey bee nest: and
(b) the stingless bee nest.
Honey bee comb cell metrics are depicted in Table 4.3.xxxi
| Cell characteristics (mm) | Apis mellifera ligustica sub genus Apis | Apis cerana ceranaxxxii sub genus Apis | Apis floreaxxxiii sub genus Micapis | Apis dorsataxxxiv sub genus Megapis |
| Worker cell width | 5.497 ± 0.004 | 4.825 ± 0.003 | 3.06 (2.8-3.2) | 5.54 ± 0.16 |
| Worker cell depth | 12.088 ± 0.040 | 9.595 ± 0.066 | 9.18 (7.5-10.0) | 16.79 ± 0.65 |
| Drone cell width | 6.626 ± 0.005 | 5.627 ± 0.009 | 4.74 (4.0-5.1) | 5.54 ± 0.16 |
| Drone cell depth | 13.776 ± 0.038 | 10.533 ± 0.144 | 13.0 (13.0) | 16.79 ± 0.65 |
| Honey cell width | 5.738 ± 0.008 | 4.922 ± 0.004 | 3.06 (2.8-3.2) | 6.25 ± 0.39 |
| Honey cell depth | 16.857 ± 0.064 | 15.499 ± 0.062 | 9.18 (7.5-10.0) | variable |
Table 4.3 Cell dimensions for representative honey bees.
Records of stingless bee cell sizes and nest structures are far and few between, those for a few well studied Australian stingless bee species are shown in Table 4.4. Unlike honey bees, where worker, drone and queen cell sizes as well as the morphology of adults are characteristically very different within species (though worker and drone cell sizes of the giant honey bees are the same size), stingless bees worker and queen cells and adults are often of the same size and adults can be difficult to distinguish.
| Cell characteristics (mm) | Tetragonula carbonariaxxxv | Tetragonula hockingsixxxvi | Tetragonula grettissixxxvii | Tetragonula claviclesxxxviii |
| Worker cell width | 2.50 | 3.35 | 4.29 ± 0.19 | 2.44 ± 0.15 |
| Worker cell depth | 3.50 | 4.00 | 5.85 ± 0.20 | 3.31 ± 0.14 |
| Queen cell width | – | – | 5.16 ± 0.24 | – |
| Queen cell depth | – | – | 8.91 ± 0.14 | – |
| Pollen pot width | – | – | 19.11 ± 0.85 | – |
| Pollen pot depth | – | – | 24.24 ± 0.32 | – |
| Honey pot width | – | – | 20.14 ± 0.39 | 6.06 ± 0.96 |
| Honey pot depth | – | – | 24.86 ± 0.21 | 7.30 ± 1.05 |
Table 4.4 Cell dimensions of Eastern Australian and one Thai Tetragonula species.
Brood of stingless bees are raised in once-only-use globular cups aggregated to form comb.xxxix Stingless bees also construct distinctive pots on the nest margin, as do bumble bees, to store pollen and honey.
In contrast honey bee worker and drone cells are constructed on a common wall hexagonal cell plan that serve to raise brood and also for storage. This design is efficient in terms of brood provisioning, rigidity and material use. Other nuanced differences between the stingless and honey bee tribes are only apparent at the species level. For example giant honey bees construct larger cells for pollen and nectar storage and raise workers and drones in the same sized cells.
Since honey and stingless bees have evolved separately their common attributes have arisen as a result convergent evolution, parallel evolution of the same traits.
Bumble bees and honey bees compared
Ye must perdoune my wyttes/ for I tell youe plaine,
I have a hive of humble bees swarmynge in my braine.
The Early English Text Society. Respublica (1553).xl
Röseler and van Honkxli provide a generalised overview of the bumble bee life system based on studies of Bombus terrestris. This species is endemic to the palearctic, a region that stretches across all of Eurasia north of the Himalayas and that includes North Africa. As already noted bumble bee queens exert less dominance over worker bees than do queens amongst honey bees. While workers in both tribes are infertile – they very rarely produce female offspring – amongst bumble bees they nevertheless play a critical role in producing drones. Indeed workers with more developed ovaries will even evict the queen and certainly compete with her in egg production and egg cannibalisation and will suppress egg laying amongst sister workers.
Bumble bee colonies in the early establishment phase that lose their queen perish. Large queenless colonies, however, stabilise under the influence of dominant workers and produce many drones. Such colonies complement queenright colonies that produce both virgin queens and drones ensuring the production of large numbers of fertile founding queens.
The caste system is nevertheless inimical to the functioning of the bumble bee colony. To the end workers nurture offspring purposed to become queens and drones. Young eclosed virgin queens play no part in colony defence or brood nurture, concentrate on building vast reserves of glycogen and lipids and finally leave the nest to mate prior to going into winter diapause.
At new season startup, the founding bumble bee queens entire focus is on establishing a nest and raising workers that will take over foraging and nest duties necessary for colony initiation. The mature colony’s function is then that of a factory to raise queens and drones to repeat the eternal cycle. xlii
In a major genomic study of the widely managed bumble bee species Bombus terrestris and Bombus impatiens Sadd and 143 coworkers highlighted honey bee and stingless bee characteristics (Table 4.5).xliii
| Attribute | Honey bee | Bumble bees | |
| Apis mellifera | Bombus impatiens | Bombus terrestris | |
| Native range | Africa/Asia/Europe | Temperate North America | Palearctic region (Europe) |
| Nesting | Cavity nesters small entrance, typically 40 L (European races of Apis mellifera) | Ground nesting | |
| Nest location | Trees, other above ground cavities | Typically nesting 30-100 cm below the surface at end of a 50-300 cm tunnel | |
| Foraging | Generalist foragers of nectar and pollen; waggle dance recruitment | Generalist but sometimes specialist pollinators, search and traplining harvesting behaviour | |
| Worker tasking | Age related with younger bees focused on brood rearing, middle aged bees on comb building and nectar processing with older bees foraging | In part size related with smaller workers attending brood while larger workers are foragers. Very young bees forage. | |
| Brood pattern | Aggregated in hexagonal comb, drone and queen cells typically on brood nest margin | Clumped and irregularly placed in nest | |
| Colony cycle | Perennial | Annual with queen diapause | |
| Colony founding | Colony fission | Solitary nest founding | |
| Sociality | Advanced (obligate permanent) eusocial | Primitively (obligate temporary) eusocial | |
| Colony size | ~20,000-100,000 | <400 | |
| Queen mating | Highly polyandrous | Limited polyandry | Monandrous |
| Worker labour division | Age-based | Size and age based | |
| Caste differentiation | Morphology, size and physiology | Size and physiology | |
| Worker reproduction (drone producing) | Rare (more common in queenless colonies) | Common | |
Table 4.5 Key differences and similarities between honeybees and bumble bees adapted from Sadd and coworkers.
Other hymenopterans
Corbiculate bees, like other social animals, have divergent approaches to managing the key challenges of group living, not least among multi queened colonies of the orchid bees. Rather than always being ‘stand alone individuals’, orchid bees, like the other corbiculates, are very often ‘stand together’ social bees. The mysteries of social organisation in the Euglossini orchid bees can be extended to the social organisations associated with the extinct corbiculate tribes, Melikertini, Electrapini and Electrobombini. The tribe Melikertini appears to have had advanced eusocial species, e.g. Mochlomelikertes hoffeinsorum (Figure 4.9).xliv

Figure 4.9 Holotype male of extinct Mochlomelikertes hoffeinsorum recovered from Baltic amber:
(a) dorsal view; and
(b) lateral view.
Beyond the inter tribal differences
With some perspective of the life systems of the different corbiculate tribes we can now turn to the more specific details of what each group represents. And we can ask searching questions about the pivotal role members of each of these communities play. How, for example, does a colony from each tribe entirely dependent on a single queen respond to her loss or poor performance? How does nest provisioning, nest architecture or nest guarding influence the success of a colony and how do strategies for establishment of new colonies line up one against the other?
In reviewing the sweat and carpenter bees we focussed on describing the different degrees of bee sociality: the gregarious (non solitary), the social, the semi social, the quasi social, the para social, the sub social and, at its zenith, the eusocial. The nature of these associations is by no means always apparent as Michener pointedly observes.xlv
When one opens a nest containing a small colony of bees, it is often impossible to recognize the relationships among the adult female inhabitants. The colony might be communal, quasisocial, or semisocial… Such colonies can be called parasocial, a noncommittal umbrella term used for a colony whose members are of a single generation and interact in any of the three ways indicated or in some as yet unrecognized way. At first, primitively eusocial colonies may look like parasocial colonies, but one individual, the queen (mother), is older, more worn, and sometimes larger than the others, which are workers (daughters). The queen commonly has enlarged ovaries and sperm cells in the spermatheca; workers usually do not.
In most respects such terminology is semantic or inadequate to fully define fully the strategic social alliances that different taxa adopt. However we can recognise that advanced social structure has produced many very successful life forms, efficient scavengers in the ants, generalist and effective pollinators and nectar gatherers in the stingless, bumble and honey bees and distinctive tropical pollinators amongst the orchid bees.
Eusociality amongst the corbiculates is singularly different to all the social arrangements that other insects have adopted. While para social species might be regarded as an association of convenience, where any cooperative behaviour is not shared between generations, corbiculate bee eusocial lifestyle confers on their number dominance in invertebrate numbers in terrestrial ecosystems. Without them we would not otherwise have honey from honey bees or ants and marauding wasps pestering us at barbeques. In Part V we will elaborate on corbiculate bee nesting and behavioural traits such as swarming to gain a clearer picture of the social systems that characterise each tribe and gain an appreciation of what they represent.
Readings
iGrüter, C. (2020). Stingless bees: Their behaviour, ecology and evolution, 395pp. Fascinating Life Sciences. https://sci-hub.mksa.top/10.1007/978-3-030-60090-7
iiCardinal, S. and Danforth, B.N. (2013). Bees diversified in the age of eudicots. Proceedings of the Royal Society B: Biological Sciences 280(1755):20122686. https://www.danforthlab.entomology.cornell.edu/wp-content/uploads/72cardinaldanforth2013prsl.pdf
iiiWikipedia (accessed 31 July 2023). Crown group. https://en.wikipedia.org/wiki/Crown_group
ivSadd, B.M., Barribeau, S.M., Bloch, G., de Graaf, D.C., Dearden, P., Elsik, C.G., Gadau, J., Grimmelikhuijzen, C.J.P., Hasselmann, M., Lozier, J.D., Robertson, H.M., Smagghe, G., Stolle, E., Van Vaerenbergh, M., Waterhouse, R.M., Bornberg-Bauer, E., Klasberg, S., Bennett, A.K., Câmara, F., Guigó, R., Hoff, K., Mariotti, M., Munoz-Torres, M., Murphy, T., Santesmasses, D., Amdam, G.V., Beckers, M., Beye, M., Biewer, M., Bitondi, M.M.G., Blaxter, M.L., Bourke, A.F.G., Brown, M.J.F., Buechel, S.D., Cameron, R., Cappelle, K., Carolan, J.C., Christiaens, O., Ciborowski, K.L., Clarke, D.F., Colgan, T.J., Collins, D.H., Cridge, A.G., Dalmay, T., Dreier, S., du Plessis, L., Duncan, E., Erler, S., Evans, J., Falcon, T., Flores, K., Freitas, F.C.P., Fuchikawa, T., Gempe, T., Hartfelder, K., Hauser, F., Helbing, S., Humann, F.C., Irvine, F., Jermiin, L.S., Johnson, C.E., Johnson, R.M., Jones, A.K., Kadowaki, T., Kidner, J.H., Koch, V., Köhler, A., Kraus, F.B., Lattorff, H.M.G., Leask, M., Lockett, G.A., Mallon, E.B., Antonio, D.S.M., Marxer, M., Meeus, I., Moritz, R.F.A., Nair, A., Näpflin, K., Nissen, I., Niu, J., Nunes, F.M.F., Oakeshott, J.G., Osborne, A., Otte, M., Pinheiro, D.G., Rossié, N., Rueppell, O., Santos, C.G., Schmid-Hempel, R., Schmitt, B.D., Schulte, C., Simões, Z.L.P., Soares, MP.M., Swevers, L., Winnebeck, E.C., Wolschin, F., Yu, N., Zdobnov, E.M., Aqrawi, P.K., Blankenburg, K.P., Coyle, M., Francisco, L., Hernandez, A.G., Holder, M., Hudson, M.E., Jackson, L., Jayaseelan, J., Joshi, V., Kovar, C., Lee, S.L., Mata, R., Mathew, T. and Newsham, I. (2015). The genomes of two key bumblebee species with primitive eusocial organization. Genome Biology 16(1):1-32. doi:10.1186/s13059-015-0623-3
vCardinal, S., and Danforth, B.N. (2011). The antiquity and evolutionary history of social behavior in bees. PLoS ONE 6(6):e21086. doi:10.1371/journal.pone.0021086 https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0021086
viCardinal and Danforth (2011) loc. cit.
viiOldroyd, B.P. and Pratt, S.C. (2015). Chapter 4, Comb architecture of the eusocial bees arises from simple rules used during cell building. In Advances in Insect Physiology 49:101-121. Academic Press. doi.org/10.1016/bs.aiip.2015.06.001 https://www.sciencedirect.com/science/article/abs/pii/S0065280615000247 https://www.public.asu.edu/~spratt1/Publications/Oldroyd%20and%20Pratt%202015.pdf
viiiZahara, I., Fahri, F., Lamerkabel, J.S., Qashiratuttarafi, Q., Juliandi, B. and Raffiudin, R. (2022). Landmark-based geometric morphometric of Apis dorsata and A. d. binghami wing venation in Indonesian archipelagos. HAYATI Journal of Biosciences 29(5):658-668. https://journal.ipb.ac.id/index.php/hayati/article/view/41390 https://www.researchgate.net/publication/361715060_Landmark-Based_Geometric_Morphometric_of_Apis_dorsata_and_A_d_binghami_Wing_Venation_in_Indonesian_Archipelagos
ixSakagami, S.F., Matsumura, T. and Ito, K. (1980). Apis laboriosa in Himalaya, the little known world largest honeybee (Hymenoptera, Apidae). Insecta Matsumurana 19:47-78. https://eprints.lib.hokudai. ac.jp/dspace/bitstream/2115/9801/1/19_p47-77.pdf
xCardinal and Danforth (2011) loc. cit.
xiPorto, D.S. and Almeida, E.A.B. (2021). Corbiculate bees (Hymenoptera: Apidae): Exploring the limits of morphological data to solve a hard phylogenetic problem. Insect Systematics and Diversity 5(3)2:1-40. https://academic.oup.com/isd/article/5/3/2/6278479?login=false
xiiWade, A. (2023). Let’s talk about wasps. The Beekeepers Quarterly 153:46-50.
xiiiGreen, C.L. and Oldroyd, B.P. (2002). Queen mating frequency and maternity of males in the stingless bee Trigona carbonaria Smith. Insectes Sociaux 49(3):196-202. doi:10.1007/s00040-002-8301-3 https://www.researchgate.net/publication/226662507_Queen_mating_frequency_and_maternity_of_males_in_the_stingless_bee_Trigona_carbonaria_Smith
xivPaxton, R.J., Weißschuh, N., Engels, W., Hartfelder, K. and Quezada-Euan, J.J.G. (1999). Not only single mating in stingless bees. Naturwissenschaften [The Science of Nature] 86(3):143-146. doi:10.1007/s001140050588 https://www.researchgate.net/publication/225756707_Not_Only_Single_Mating_in_Stingless_Bees
xvEngel, M.S. (2001). A monograph of the Baltic amber bees and evolution of the Apoidea (Hymenoptera). Bulletin of the American Museum of Natural History 259:1-192. https://www.biodiversitylibrary.org/item/320390#page/1/mode/1up
xviCardinal, S. and Danforth, B.N. (2011) loc. cit.
xviiEngels and Imperatriz-Fonseca (1990) loc. cit. p.168.
xviiiRomiguier, J., Cameron, S.A., Woodard, S.H., Fischman, B.J., Keller. L. and Praz, C.J. (2016). Phylogenomics controlling for base compositional bias reveals a single origin of eusociality in corbiculate bees. Molecular Biology and Evolution 33(3):670-678. https://www.semanticscholar.org/paper/Phylogenomics-Controlling-for-Base-Compositional-a-Romiguier-Cameron/e6f10dd12b36d301cac0a2229a4198932693dffe
Bossert, S., Murray, E.A., Blaimer, B.B. and Danforth, B.N. (2017). The impact of GC bias on phylogenetic accuracy using targeted enrichment phylogenomic data. Molecular Phylogenetics and Evolution 111(1):149-157. https://www.sciencedirect.com/science/article/abs/pii/S1055790317301033?via%3Dihub doi:10.1016/j.ympev.2017.03.022
Bossert, S., Murray, E.A., Almeida, E.A.B., Brady, S.G., Blaimer, B.B. and Danforth B.N. (2019). Combining transcriptomes and ultraconserved elements to illuminate the phylogeny of Apidae. Molecular Phylogenetics and Evolution 130(1):121-131. https://www.semanticscholar.org/paper/Combining-transcriptomes-and-ultraconserved-to-the-Bossert-Murray/ccaf3ef7cfd2354a1c77ee5884f0aa408d9fb9d3 doi:10.1016/j.ympev.2018.10.012
xixEngels, W. and Imperatriz-Fonseca, V.L. (1990). Chapter 7. Caste development, reproductive strategies, and control of fertility in honey bees and stingless bees. In Engels, W. (ed). Social insects: An evolutionary approach to castes and reproduction. pp.167-230. Springer, Berlin, Heidelberg. https://sci-hub.se/10.1007/978-3-642-74490-7_9 https://link.springer.com/chapter/10.1007/978-3-642-74490-7_9 For a broader review of the complex ecology of these two eusocial tribes see Roubik, D.W. (2023). Stingless bee (Apidae: Apinae: Meliponini) ecology. Annual Review of Entomology 68(1):231-256. https://www.annualreviews.org/doi/10.1146/annurev-ento-120120-103938
xxMöbus, B. (1998). Brood rearing in the winter cluster, Part I. American Bee Journal 138(7):511-514. https://scientificbeekeeping.com/ scibeeimages/Mobus-1998-brood-rearing-in-winter-cluster.pdf.
Möbus, B. (1998). Rethinking our ideas about the winter cluster, Part II. American Bee Journal 138(8):587-591. https://scientificbeekeeping.com/ scibeeimages/Mobus-1998-winter-cluster-part-2.pdf
xxiTan, K., Yang, S., Wang, Z-W., Radloff, S. and Oldroyd, B. (2012). Differences in foraging and broodnest temperature in the honey bees Apis cerana and A. mellifera. Apidologie 43(6):618-623. https://hal.science/hal-01003658/file/hal-01003658.pdf
https://www.tib.eu/en/search/id/BLSE%3ARN321836196/Differences-in-foraging- and-broodnest-temperature/
xxiiKastberger, G., Waddoup, D., Weihmann, F. and Hoetz, T. (2016). Evidence for ventilation through collective respiratory movements in giant honeybee (Apis dorsata) nests. PLoS One 11(8):e0157882.
doi:10.1371/ journal.pone.0157882
xxiiiArias M.C. and Sheppard, W.S. (2005). Phylogenetic relationships of honey bees (Hymenoptera:Apinae:Apini) inferred from nuclear and mitochondrial DNA sequence data. Molecular Phylogenetics and Evolution 37(1):25–35. doi:10.1016/j.ympev.2005.02.017 https://www.ncbi.nlm.nih. gov/pubmed/16182149
xxivGrüter (2020) loc. cit.
xxvGrüter (2020) loc. cit.
xxviMelo, G.A.R. (2020). Stingless bees (Meliponini). In Starr, C.K. (ed). Encyclopedia of social insects. pp.883-900. https://sci-hub.mksa.top/10.1007/978-3-030-28102-1_117 doi: 10.1007/978-3-030-28102-1_117 https://link.springer.com/referenceworkentry/10.1007/978-3-030-28102-1_117 Springer International, Cham.
xxviiAntonelli, A., Zizka, A., Carvalho, F.A., Scharn, R., Bacon, C.D., Silvestro, D. and Condamine, F.L. (2018). Amazonia is the primary source of Neotropical biodiversity. Proceedings of the National Academy of Sciences 115(23):6034-6039. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6003360/
xxviiiBorst, P. (May 2015). The origin and distribution of honey bees. American Bee Journal 155(5):565-568. https://www.researchgate.net/publication/298082768_The_origin_and_distribution_of_honey_bees
Grüter, C. (2020). loc. cit.
xxixSeeley, T.D. and Morse, R.A. (1976). The nest of the honey bee (Apis mellifera L.). lnsectes Sociaux 23(4):495-512. doi:10.1007/BF02223477 http://www.naturalbeekeeping.com.au/Nest%20of%20the%20 Honeybee,%20Seeley%20and%20Morse.pdf https://www.researchgate. net/publication/269996264_The_nest_of_the_honey_bee_Apis_ mellifera_L
xxxMichener, C.D. (2003). Chapter 1. pp.1-17. The Meliponini. In Vit, A.P., Pedro, S.R.M. and Roubik, D.W. (eds). (2013). Pot-Honey: A legacy of stingless bees. 648pp. Springer, New York, Heidelberg, Dordrecht, London.
xxxiWade, A. (2023). The honey bee comb catacomb. The Beekeepers Quarterly 151:38-42.
xxxiiYang, S., Deng, S., Kuang, H., Zhou, D., Gong, X. and Dong, K. (2021). Evaluating and comparing the natural cell structure and dimensions of honey bee comb cells of Chinese bee, Apis cerana cerana (Hymenoptera: Apidae) and Italian bee, Apis mellifera ligustica (Hymenoptera: Apidae). Journal of Insect Science 21(4):1-8. https://academic.oup.com/jinsectscience/article/21/4/1/6313200?login=false
xxxiiiKhan, M.S., Kaushik, H.d. and H. R. Rohilla, H.R. (2002). Nesting parameters and comb dimensions of the dwarf honeybee, A. florea F. at Hisar, India. Indian Bee Journal 64(162):12-19. https://www.researchgate.net/publication/327386902_Nesting_parameters_and_comb_dimensions_of_the_dwarf_honeybee_A_florea_F_at_Hisar_India
xxxivBuawangpong, N., Saraithong, P., Khongphinitbunjong, K., Chantawannakul, P. and Burgett, M.D. (2014). The comb structure of Apis dorsata F. (Hymenoptera: Apidae): 3-dimensional architecture and resource partitioning. Chiang Mai Journal of Science 41(5):1077-1083. https://www.thaiscience.info/journals/Article/CMJS/10932954.pdf
xxxvBrito, R.M., Schaerf, T.M., Myerscough, M.R., Heard, T.A. and Oldroyd, B.P. (2012). Brood comb construction by the stingless bees Tetragonula hockingsi and Tetragonula carbonaria. Swarm Intelligence 6(2):151-176. doi:10.1007/s11721-012-0068-1
xxxviDollin, A.E. and Dollin, L.J., (1997). Australian stingless bees of the genus Trigona (Hymenoptera: Apidae). Invertebrate Systematics 11(6):861-896. CSIRO Publishing. https://www.researchgate.net/publication/248899861_Australian_stingless_bees_of_the_Genus_Trigona_Hymenoptera_Apidae
xxxviiChauhan, A. and Singh, H.K. (2021). Nest architecture of stingless bee, Tetragonula gressitti Sakagami from Nagaland, India. International Journal of Tropical Insect Science 41:3099-3104.doi:10.1007/s42690-021-00503-w https://link.springer.com/article/10.1007/s42690-021-00503-w
xxxviiiChuttong, B. and Burgett, M. (2017). Biometric studies of the stingless bee Tetragonula clavicles complex (Apidae: Meliponini) from Northern Thailand. Journal of Apiculture 32(4):359-362. http://journal.bee.or.kr/xml/12194/12194.pdf
xxxixOldroyd, B.P. and Pratt, S.C. (2015). Chapter 4. Comb architecture of the eusocial bees arises from simple rules used during cell building. In Advances in Insect Physiology 49:101-121. Academic Press. doi.org/10.1016/bs.aiip.2015.06.001 https://www.sciencedirect.com/science/article/abs/pii/S0065280615000247 https://www.public.asu.edu/~spratt1/Publications/Oldroyd%20and%20Pratt%202015.pdf
Oldroyd, B.P. (2020). Dwarf honey bees (Apis (Micrapis)). In Starr, C.K. (ed). Encyclopedia of social insects. pp.333-339. doi: 10.1007/978-3-030-28102-1_117
xlThe Early English Text Society Original series 226 (1952 for 1946). In Greg, W.W. (ed). Respublica: An interlude for Christmas 1553 attributed to Nicholas Udall. Prologue. p.3. https://archive.org/details/respublicaearlye0000wwgr/page/3/mode/1up
xliRöseler, P.F. and van Honk, C.G. (1990). Castes and reproduction in bumblebees. In Social insects: An evolutionary approach to castes and reproduction. pp.147-166. Berlin, Heidelberg: Springer Berlin Heidelberg. https://link.springer.com/chapter/10.1007/978-3-642-74490-7_8 doi:10.1007/978-3-642-74490-7_8
xliiMichener, C.D. (1974). The social behavior of the bees: A comparative study. Harvard University Press. Part III, Chapter 28 Bumble bees pp.314-328. https://archive.org/details/socialbehaviorof0000mich/page/314/mode/1up
xliiiSadd and coworkers (2015) loc. cit.
xlivEngel, M.S. Breitkreuz, L.C.V. and Ohl, M. (2014). The first male of the extinct bee tribe Melikertini (Hymenoptera: Apidae). Journal of Melittology 30:1-18. https://journals.ku.edu/melittology/article/view/4698 doi:10.17161/jom.v0i30.4698
xlvMichener, C.D. (2007). The Bees of the World, second edition. The Johns Hopkins University Press, Baltimore, Maryland. Section 5. Solitary versus social life. p.13.
