
Muttabuttasaurus when the first social bees emerged.
Australian National Museum, 16 June 2023.
Photo: Alan Wade
Alan Wade
In the beginning
In the beginning were the insects:
And the Lord said unto Moses, Stretch out thine hand over the land of Egypt for the locusts, that they may come up upon the land of Egypt, and eat every herb of the land, even all that the hail hath left.
Exodus 10:12
The hymenoptera, the orthoptera and all the other insects have come in their countless billions. And they all came into being many millions, not thousands, of years ago. The Orthoptera became the locusts, the crickets, the grasshoppers and cicadas, all solitary insects that are each unimaginably destructive. The ancestral hymenopteran similarly shook off its shackles and also spawned the ants, assorted wasps and bees. Their phylogeny is based on an interpretation of morphologies and genetic makeup of taxaa that we have been been able to study. Boiled down they arose from three aculeate superfamilies, the Apoidae (the bees), the Vespoidae (the stinging wasps and an early spinoff ant clade) and the Sphecidae (the sand, mud dauber and thread-waisted wasps). Their exact origins are unknowable.i
But how and why did a few bees – and some wasps and all the ants – conjure up the trick of forming social compacts? How did the solitary insect or bee transition to an advanced society where several generations lived together with every member of the community pitching in? After all we now have hives of bees working meadows and trees in blossom to harvest, process and store prodigious amounts of honey and nutritious pollen.
Bees can be social to the extent that they simply congregate and where each female continues to produce her own offspring. More advanced forms of sociality emerged where there was division of labour amongst the female members of the community, a female skew that in various measure put apart the laying queen from her workers. The latter carried out most if not all of the nest provisioning and brood nurturing tasks. Such highly organised communities are deemed eusocial.
Perhaps the most enlightened discourse on the existence of eusocial bee societies comes from Charles Michener.ii He emphasises that the term eusocial should be related to a colony and not to a species. For example many species of bees can go down either a solitary pathway or nest as a eusocial colony. Such species are deemed facultatively eusocial.
Beyond a primitively facultative eusocial existence there are two advanced alternatives:
- the obligate temporary eusocial colony where a foundress queen must undertake all nest duties in establishing new colonies as displayed by most bumble bees; and
- the fully obligate eusocial colony where a queen and ‘helper’ workers are always present – the stingless and honey bees.
As Michener further observed:
A colony consists of two or more adult females, irrespective of their social relationships, living in a single nest. Frequently the females constituting a colony can be divided into:
- one to many workers which do most or all of the foraging, brood caring, guarding, etc., and are often unmated; and
- one queen, who does most or all of the egg laying and is usually mated.
The queen is often, and in some species always, larger than her workers, but sometimes the difference is only in mean size.
The queen is often, and in some species always, larger than her workers, but sometimes the difference is only in mean size.
We can adjudge how extremely rare eusociality is amongst different types of bees, if not amongst the ants and wasps, from the chart (Figure 1.1), a veritable maze. The eusocial hot spots are shown in blue. The phylogenetic tree includes the large bee family Apidae with its corbiculate bees as well as the Xylocopinae carpenter bees and the also very large family Halictidae with its sweat bees, many of which are social in some measure and a number of which are eusocial. To cut to the quick, two families and several tribes contain species that mimic much of what honey, stingless and bumble bees are capable of. We will come back to the timelines that overarch the emergence of the ants, bee and wasp families, but first let us explore what sociality and eusociality actually encompass.
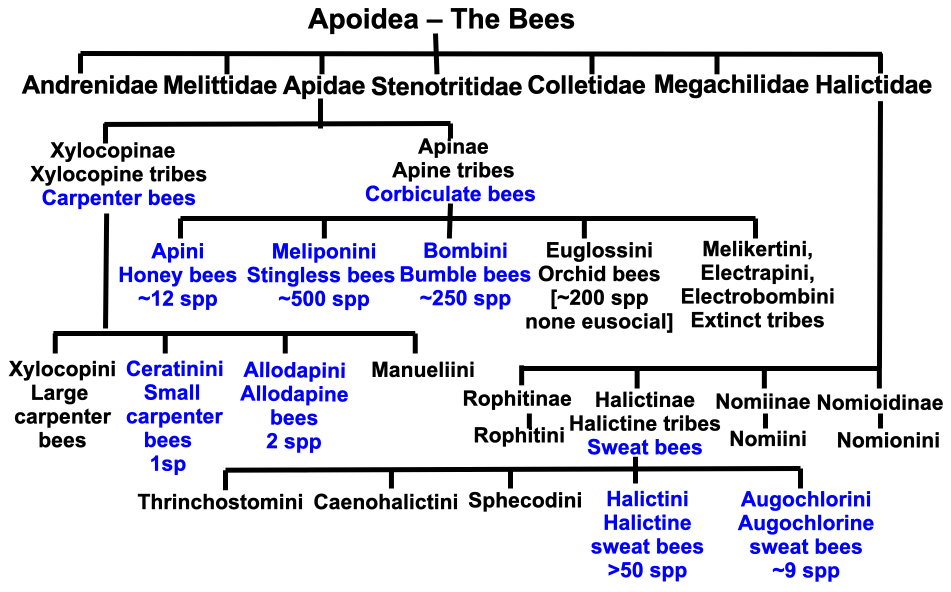
Figure 1.1 Social bee chart showing tribes depicted in blue containing several to many eusocial species. The lost tribes Melikertini, Electrapini and Electrobombini were more than likely eusocial but we only have their fossil record.
Social and eusocial insect behaviour comes in many colours, the most obvious sort seen in honey, stingless and bumble bees, in ant colonies, and in some wasps, where queen and worker bee castes are an almost permanent fixture.
Obligate permanent eusociality
The notions of socially sophisticated, multigenerational colonies of honey bees, stingless bees and ants enjoying a jolly life is ingrained in our collective consciousness. If you listen extremely carefully at the next family picnic you are sure to hear the ants weighing up their options:
‘Shall we eat him here?’
or
‘Shall we take him home with us?’
Yet harnessing the services of short-lived worker bees attending to a long-lived queen, each servicing each others needs and each being dependent on each other – toss in a few drones – has been a remarkable achievement. Such constant cooperation demonstrated by these sophisticated hymenopterans is termed permanent obligate eusociality.
Short of the whole colony dying, the community – rather than the individual – is a permanent fixture. The corbiculate honey and stingless bees fit this category and are joined by a few species of wasp (e.g. the Yellow brown paper wasp Ropalidia romandi and the night flying Provespa wasps) and all of the separately evolved ant family. They all form permanent nests and reproduce by swarming or, in the case of some ants, also by budding off or by releasing some reproductives.
Obligate temporary eusociality
There is a lesser form of eusocial behaviour. Many wasps, bumble bees and other social insects are ever only temporarily eusocial: the community is not a permanent fixture. In these, gynes (potential reproductive females) and drones (males) are produced en masse towards the close of each season when the colony dies. Single foundress queens establish new colonies each spring or, in warmer climes, in follow up breaking rains.
With temporary eusociality the process begins with the queen foraging and raising brood alone. However she soon recruits her daughters to do the housework, nurse larvae, take out the garbage and have them head out to earn the family a living. This scheme leaves it to mum to produce new babies, rather lay eggs, that upon hatching her daughters nurture and incubate. Against seemingly daunting odds, the colony – as a single entity – gradually expands and, under favourable conditions, flourishes. Then, unlike honey and stingless bees that switch to a strategy of storage, the colony commences building progressively larger cells, in them raising large numbers of queens and drones. The cycle is then repeated.
Yet this facility is sometimes lost, exemplified by parasitic bumble bees and the orchid bees whose progenitors were primitively eusocial but whose species, while primarily solitary, sometimes behave socially.
Facultative eusociality
There is one other type eusocial behaviour, likely the progenitor of an obligate eusocial lifestyle, termed facultative eusociality. Here, after mating, the queen can behave just like any other solitary insect, that is raise brood on her own and never establish a colony. Under favourable conditions, however, she may instead form a colony. Like obligately eusocial bees and some wasps, the queen then co-opts offspring that form a well-defined, functionally sterile – at least unmated – worker caste. And like obligately eusocial bees, these workers forage and attend to nest duties to increase the queen’s reproductive success.
This plasticity in behaviour – solitary or social existence – is often governed by environmental factors, typically length of season, latitudinal limits or elevation where the advantages conferred by social cooperation are demonstrably lost. To complicate matters, there are instances where a mixture of solitary and eusocial nests of a single species can be found in the one locality, perhaps reflected in genetic variability or the sort of start an individual queen makes.
Notably this switching facility is not available to obligately eusocial ants, wasps and bees. Obligately eusocial insects have passed the point of no return. They cannot revert to the solitary condition, that is reproduce and survive entirely on their own.
Community or aggregate sociality
There are other simpler forms of social behaviour – para social, semi social, sub social, communal – where each queen (all females) raises her own offspring. Here – where there is never a worker caste – some sharing of resources, say a nesting cavity or defendable entrance, works to the benefit of all members of the community. It is predicated on the safety in numbers principle. For ground nesting insects, for example, sharing a common vertical tunnel – where each of a number of queens has its own separate nesting side chamber – confers a collective advantage. Such aggregate behaviour may equally work against the success of individual reproductives: a less fit female may lose out to a female that usurps her pollen and nectar store, a mild form of kleptoparasitism.
Other seemingly more benign assemblages, massed sawfly larvae or large numbers of male blue banded bees perched together may confer some reproductive advantage to the species. For all practical purposes, however, these behaviours are largely those of solitary insects.
We now turn to examining how these social conditions arose.
Evolution of eusociality in the hymenopterans
Social bees and wasps raise their young in well defined cell cups while other social insects, such as the ants and termites, have protective nests and defined chambers to raise their offspring.
Fingerprints of social origin
Contemporary clues as to how such cooperative behaviour – and shared nest building – arose comes mainly from the extremely diverse halictid bee family and its halictine sub families. As Richards and Wessberg outline succinctly:iii
Halictine sweat bees (Hymenoptera: Halictidae) exhibit an astonishing range of social behavior and are significant model organisms for studying the evolution of altruism and eusociality. Most species are solitary, with female bees constructing nests and raising their own brood, but there are also many types of colony social organisation, ranging from more or less egalitarian communal societies, to semi social and eusocial types, in which more or less sterile workers aid queens that dominate reproduction. The differences between communal and eusocial halictine societies are fundamental.
Richards and Wessberg go on to outline the key differences between the community, where the individual insect retains most of its independence in a social setting, and the eusocial condition, where near sterile female workers sacrifice their reproductive potential instead foraging and undertaking nest duties:
First, communal societies do not have queen and worker castes, being composed of multiple females that may or may not be related, and exhibiting a low degree of reproductive skew and little aggression among nest mates. Eusocial societies are characterized by queen and worker reproductive castes with a high degree of reproductive skew (queens lay all or most of the eggs) and substantial aggression, especially by [the] queens toward workers. Second, communal societies are composed of adults of the same generation, whereas eusocial societies are formed when a queen’s offspring become workers.
The social bee and wasp nests comprise either a loose assemblages of individual, usually globular, capsules, abutted cell aggregates or, as we have observed, rigid reusable comb constructed on the hexagonal cell pattern. An extreme example is the social paper bag wasp, Ropalidia plebeiana, that dissects old comb at start up each spring: foundress queens then employ these starter comb patches to construct numerous contiguous nests.iv
Mikát, Fraňková, Benda and Strakav have scoped the spattering of socially polymorphic (facultatively social) bee tribes. As noted the largest lineages are found in just two bee families: the Halictidae (the sweat bees in the sub family Halictinae: Halictini, Augochlorini) and the Apidae (the Xylocopinae (the carpenter bees: Xylocopini, Allodapini, Ceratinini) and the Apinae (the apine bees: Apini, Meliponini, Bombini, Euglossini, the latter while, never eusocial, is nevertheless not always strictly solitary)) (Figure 1.2).vi
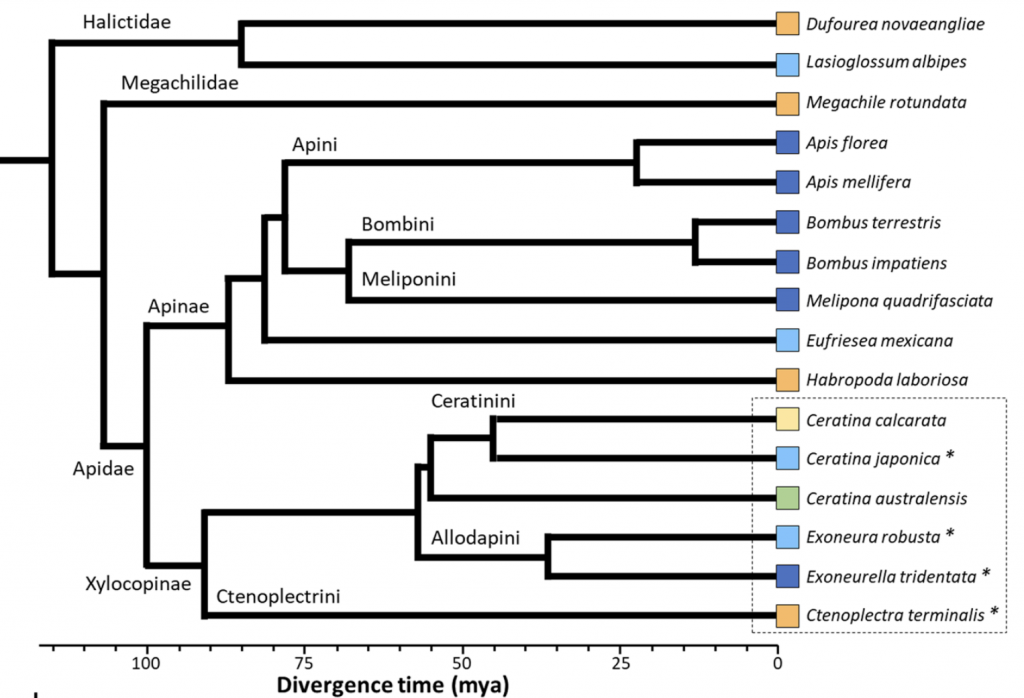
Figure 1.2 Ancestral emergence of bee families, tribes and a few representative genera and species.
Of the social tribes, the xylocopine and allodapine carpenter and ceratinine small carpenter bees (Xylocopinae: Xylocopini, Allodapini and Ceratinini) arose most recently (~35-50 mya), while the halictine sweat bees (Halictinae: mainly Halictini and Augochlorini) and the apine corbiculate bees (Apinae: Apini, Meliponini, Bombini and Euglossini) are of very ancient lineage (~95 mya) noting that the vespid (Vespidae: Vespinae; Polistinae; Stenogastrinae) wasps are of similarly early origin (~ 80 mya). In examining how theories of social behaviour arose, Miriam Richards notes:vii
The evolutionary transition from solitary to social behaviour has long been one of the central obsessions of social insect biologists. A difficulty with studying this transition is that in many social insect lineages, transitions to sociality took place tens of millions of years ago, obscuring the behavioural changes involved in these transitions. The eusocial sweat bees used to be considered an exception to this rule, because we thought that eusociality was quite recent and that there were many origins of eusociality in this group. Alas, phylogenetic research since then has conclusively demonstrated that sweat bees, like other social insect lineages, made the transition to eusociality too long ago to regard current behaviour as representing the first stages of social transitions.viii, ix
To these halictid (sweat bees) Richards adds the carpenter bees (tribes Allodapini and Ceratinini) that sometimes exhibit this conditional eusocial behaviour and the sometimes social corbiculate orchid bees (Apinae: Euglossini):
Fortunately, over the past few decades, behavioural researchers have uncovered many examples of socially polymorphic sweat bees, in which solitary or eusocial behaviour is facultatively expressed within or between populations, starting with the discovery of reversion to solitary behaviour in a high altitude population of Lasioglossum calceatum [a halictid sweat bee]x. Social polymorphism has now been identified in multiple species of sweat bees, small and large carpenter bees, and orchid bees, allowing biologists to compare both causes and consequences of solitary and eusocial behaviour, shedding empirical light on what would, otherwise, remain purely theoretical studies on the origin of social behaviour.
Evolution of eusociality in the hymenopterans
The hymenopteran insect order originated in the Lower (early) Triassic, 247-253 million years ago (mya), a period in which the purely solitary sawflies (Symphyta) split off from the the other suborder Apocrita comprising separate families of sphecid bees (Sphecidae) and the Crabronidae that in turn split into the fully eusocial ant family (Formicidae) in the Jurassic (140 and 168 mya) and into a sub clade, Aculeata (the stinging wasps and later bees) with barbed ovipositors. From the Aculeata emerged the modern super families of stinging wasps (Vespoidae), chrysid cuckoo wasps (Chysidoidae/Chrysididae) and the bees (Apoidae). The earliest fossil bee record is that of the halictid Cellicalichnus krausei (Figure 1.3).
In summary I have attempted to map out the main features and distribution of honey bee families (Table 1.1) highlighting a few of the more common genera and noting where some form of eusociality occurs. All families and most tribes comprise predominantly solitary species, eusociality being of rare occurrence.
| Family | Common name | Genera [species] | Provenance | Nest | Social status [Origin mya] |
| Stenotritidaexi | Stenotritids | Total of 21 species Ctenocolletes [10] Stenotritus [11] | Australia | Lined burrows to 3 m | Solitary [~92] |
| Colletidae | Plasterer bees | Over 880 species Colletes [~700] | Global excluding Australia, SE Asia | Underground, sometimes aggregated | Solitary [~95} |
| Apidae: Apinaexii | Bumblebees, honey bees, stingless bees, orchid bees, cuckoo bees… | About 6000 species Apis [12] Bombus [~100] some parasitic Tetragonula [31] Austroplebeia [5] | Cosmopolitan | Social species often cavity dwelling | Main repository of obligate eusocial species (Apini, Meliponini and Bombini). Most bees solitary [78-95] |
| Apidae: Xylocopinaexiii | Carpenter bees | About 500 Exoneurella [4], one Australian species eusocial | Cosmopolitan | Typically decaying wood | A few facultatively eusocial species (Xylocopini, Ceratini, Allodapini) [78-95] |
| Megachilidae | Leafcutter, mortar, carder, resin bees | About 4000 species Megachile [1520] | Cosmopolitan | Soil and wall cavities, plant stems | Solitary [67-97] |
| Halictidaexiv | Sweat and reed bees | About 4500 species Halictus [200] Lasioglossum [1800] | Cosmopolitan | Mainly ground nesting e.g. clay, stream banks | Rich collection of facultatively eusocial species [Halictini, Augochlorini] [35] |
| Melittidae | Mellitid bees | 200 species | Mainly southern Africa | Ground nesting | Solitary [53] |
| Andrenidae | Mining bees | 3000 species | North America, South America, Europe, Africa | Ground nesting | Solitary [32] |
Table 1.1 Defining bee family attributes.xv
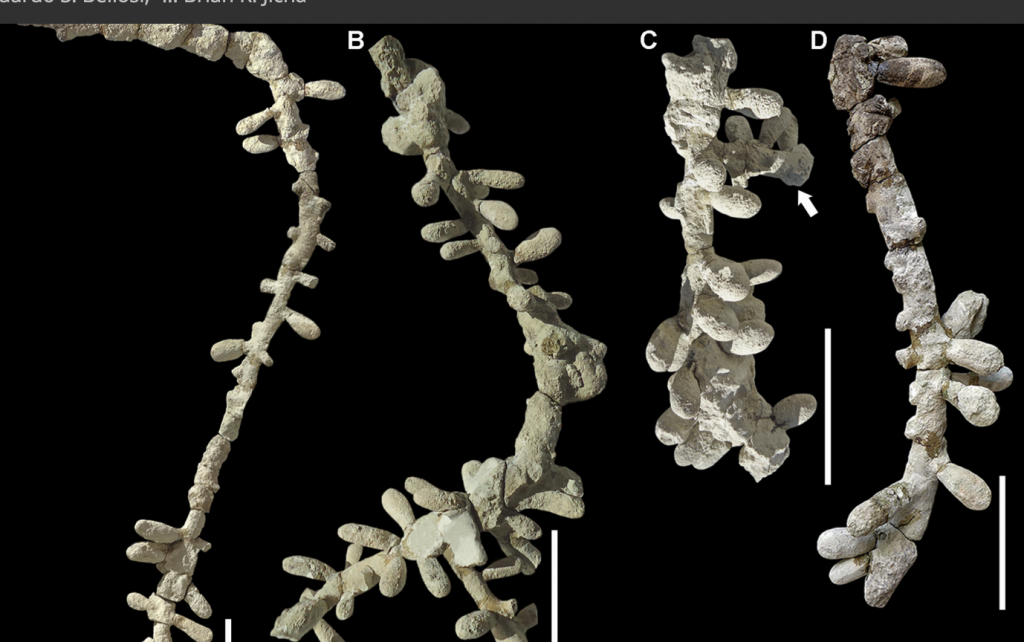
Figure 1.3 Fossilised, one hundred million year old nest of the sweat bee Cellicalichnus krausei isp. nov.xvi
In Part I of the series we have examined an array of social behaviours amongst bees. In Part II we will examine the highly social Halictidae sweat bees, in Part III the Apidae (Xylocopinae) carpenter bees and in concluding Parts IV and V the Apidae (Apinae) corbiculate bees.
Readings
iBrothers, D.J. (2021). Aculeate hymenoptera: Phylogeny and classification. In Starr, C.K. (ed). Encyclopedia of social insects. pp.3-11. https://sci-hub.mksa.top/10.1007/978-3-030-28102-1_117 doi: 10.1007/978-3-030-28102-1_117 https://link.springer.com/referenceworkentry/10.1007/978-3-030-28102-1_117 Springer International, Cham.
Brothers, D.J. (1975). Phylogeny and classification of the aculeate Hymenoptera, with special reference to Mutillidae. The University of Kansas Science Bulletin 50(2):483-648. https://archive.org/details/ants_05096/5096/
iiMichener, C.D. (2007). The Bees of the World, second edition. The Johns Hopkins University Press, Baltimore, Maryland. Solitary versus social life. Section 5. pp.12-15.
iiiRichards, M.H., von Wettberg, E.J. and Rutgers, A,C. (30 May 2003). A novel social polymorphism in a primitively eusocial bee. Proceedings of the National Academy of Sciences 100(12):7175-7180. https://www.pnas.org/doi/10.1073/pnas.1030738100 doi/10.1073/pnas.1030738100w
ivTsuchida, K., Ishiguro, N., Saito-Morooka, F., Kojima, J. and Spradbery, P. (2022). Nepotistic colony fission in dense colony aggregations of an Australian paper wasp. Scientific Reports 12:12868. https://www.nature.com/articles/s41598-022-17117-y
doi.org/10.1038/s41598-022-17117-y
vMikát, M., Fraňková, T., Benda, D. and Straka, J. (2022). Evidence of sociality in European small carpenter bees (Ceratina). Apidologie 53(2):18. https://doi.org/10.1007/s13592-022-00931-8 https://link.springer.com/article/10.1007/s13592-022-00931-8#citeas
viShell, W.A., Steffen, M.A., Pare, H.K., Seetharam, A.S., Severin, A.J., Toth, A.L. and Rehan, S.M. (2021). Sociality sculpts similar patterns of molecular evolution in two independently evolved lineages of eusocial bees. Communications Biology 4:253. doi:10.1038/s42003-021-01770-6
viiRichards, M.H. (2019). Socially polymorphic bees as model organisms for studying the evolution of eusociality. Insectes Sociaux 66(1):3-4. https://link.springer.com/article/10.1007/s00040-019-00689-w doi.org/10.1007/s00040-019-00689-w
viiiBrady, S.G., Sipes, S., Pearson, A. and Danforth, B.N. (2006). Recent and simultaneous origins of eusociality in halictid bees. Proceedings of the Royal Society B: Biological Sciences 273(1594):1643-1649. doi.org/10.1098/rspb.2006.3496
ixGibbs, J., Brady, S.G., Kanda, K. and Danforth, B.N. (2012). Phylogeny of halictine bees supports a shared origin of eusociality for Halictus and Lasioglossum (Apoidea: Anthophila: Halictidae). Molecular Phylogenetics and Evolution 65(3):926939. doi:10.1016/j.ympev.2012.08.013
xSakagami S.F. and Munakata, M. (1972). Distribution and bionomics of a transpalaeartic eusocial halictine bee, Lasioglossum (Evylaeus) calceatum, in northern Japan, with reference to its solitary life cycle at high altitude. Journal of the Faculty of Science Hokkaido University Ser. 6 (Zoology) 18:411-439. https://eprints.lib.hokudai.ac.jp/dspace/bitstream/2115/27540/1/18(3)_P411-439.pdf
xiWikipedia (accessed 25 May 2023). Stenotritidae. https://en.wikipedia.org/wiki/Stenotritidae
xiiCardinal, S., Straka, J. and Danforth, B.N. (2010). Comprehensive phylogeny of apid bees reveals the evolutionary origins and antiquity of cleptoparasitism. Proceedings of the National Academy of Sciences 107(37):16207-16211. doi:10.1073/pnas.1006299107
xiiiSakagami, S.F. and Michener, C.D. (1987). Tribes of Xylocopinae and origin of the Apidae (Hymenoptera: Apoidea). Annals of the Entomological Society of America 80(3):439-450. doi:10.1093/aesa/80.3.439
xivDanforth, B.N., Conway, L. and Ji, S. (2003). Phylogeny of eusocial Lasioglossum reveals multiple losses of eusociality within a primitively eusocial clade of bees (Hymenoptera: Halictidae). Systematic Biology 52(1):23-36. doi.org/10.1080/10635150390132687
xvMichener, C.D. (2007) loc. cit.
Museum of the Earth (accessed 25 May 2023). Bee diversity and taxonomy. https://www.museumoftheearth.org/bees/diversity
xviGenise, J.F., Bellosi, E.S., Sarzetti, L.C., Krause, JM., Dinghi, P.A., Sánchez, M.V., Umazano, A.M., Puerta, P., Cantil, L.F. and Jicha, B.R. (2020). 100 Ma sweat bee nests: Early and rapid co-diversification of crown bees and flowering plants. PLOS ONE 15(1):e0227789– doi:10.1371/journal.pone.0227789
