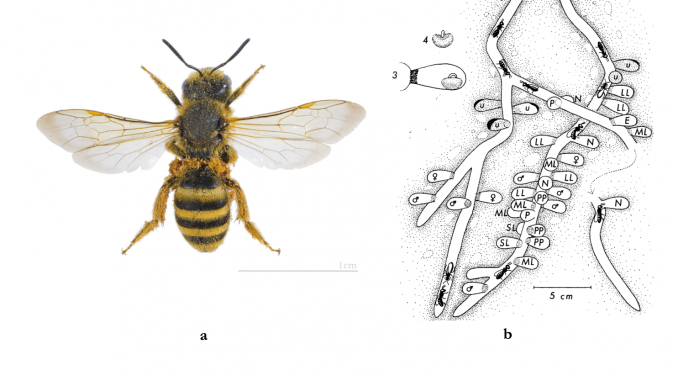
Facultatively eusocial great banded furrow-bee Halictus scabiosae:
(a) female with pollen on whole body, Toulouse, France;i and
(b) contiguous 1-5 queen nests, Geneva, Switzerland.ii
Alan Wade
Social bees, not honey bees
Sometimes we might ponder about how we ever came to keep honey bees. But do we really own them or do they, figuratively, own us? Instead we might ask how on earth bees came to be. To explain their existence we can safely put aside the spontaneous generation theory:i
…the hypothetical process by which living organisms develop from nonliving matter; also, the archaic theory that utilized this process to explain the origin of life.
That said science has been slow to the rescue in explaining the origin of highly social bees. Let us try to answer a simpler question:
How did bees learn to become social and how did they learn to construct such marvellous nests?
For starters, we simply can’t time warp back tens of millions of years to observe how the solitary bee acquired the necessary skills to cooperate and to construct a shared nest. As we have observed one bee group outside the familiar Apidae family – containing the likes of honey, stingless and bumble bees – is the Halictidae sweat bee family. It has proved to be fertile ground for unlocking the secrets of the evolutionary origins of sociality. There are nearly 45,000 sweat bees and their social taxa sure know how to party. To the mix we can throw in the Xylocopinae carpenter bee sub family, with about 500 species. It, too, displays an extraordinary range of social development. As Swartz, Richard and Danforth have noted:ii
Until the 1980s theories of social insect evolution drew strongly on halictine [Halictinae sweat] and allodapine [Xylocopinae carpenter] bees. However, that early work suffered from a lack of sound phylogenetic inference and detailed information on social behavior in many critical taxa. Recent studies have changed our understanding of these bee groups in profound ways. It has become apparent that forms of social organization, caste determination, and sex allocation are more labile and complex than previously thought, although the terminologies for describing them are still inadequate. Furthermore, the unexpected complexity means that many key parameters in kin selection and reproductive skew models remain unquantified…
At the same time, phylogenetic questions have become more tractable, and DNA sequence-based studies have resolved questions that earlier studies could not resolve, radically changing our understanding of the number of origins and losses of sociality in these bees.
What we do know is that our common or garden honey bees and their close relatives are of Cretaceous aka dinosaur origin. Their tell-all ancestors have long disappeared so they give us no insight into how social life arose. Thankfully modern day halictid and xylocopinid (sweat and carpenter) bees often adopt a flexible social lifestyle providing us clues as to the origin of social behaviour. Here factors such as latitude or height above sea level, as well as length of season, come into play. In his introduction to reproduction and castes in the social halictine sweat bees, Micheneriii surmises that:
Parasocial and primitively eusocial bees are found in various families of the Apoidea, but the majority of such forms are in the Halictinae (Family Halictidae, the sweat bees). The Halictinae are an enormous and abundant group, worldwide in distribution, arctic to tropical, and every continent has forms whose social biologies remain unknown. Although a few species nest in rotting wood, most make burrows in the soil. New and interesting types of social organization probably remain to be discovered in this subfamily, for only a tiny fraction of the species have been studied behaviorally.
Charles Michener delineates two common types of eusocial hierarchy amongst halictine sweat bees:
- temporary obligately eusocial species. Here colony foundresses undergo diapause, at least in temperate climes, and establish new colonies with a foundress queen and auxiliary workers of variable (sister or daughter) origin; and
- permanent obligately eusocial species. Here colonies are non-foundress (there is no stand-alone founding queen and there is no need for queens to undergo diapause). Queens in existing colonies produce daughter workers and replacement queens.
For a very readable account of bee social life check out Charles Michener’s book The social behavior of the bees.iv In a later follow up discourse on the Xylocopinae carpenter bee sub family – spanning the Xylocopini, Ceratinini and Allodapini tribes – Michener states that:v
[The] Xylocopinae are not usually thought of as having castes and two of the three major included tribes are not or scarcely mentioned in accounts of social behavior in bees. The only Xylocopine tribe treated in detail … [were] called simply the allodapine group of Ceratinini. We now know that some species in the other tribes are also, in varying degrees, social. Such species have, in some nests, two or more adult females with division of activities among them and sometimes behaviorally recognizable castes.
With a now very clear delineation of where truly eusocial tribes (bees with a distinct reproductive and worker castes) lie within the bee kingdom (Figure 2.1), we can explore the ancient origins of eusociality and their intricate nests. This chart separates the obligatory eusocial corbiculate tribes from the facultatively eusocial tribes that occur with variable frequency elsewhere.
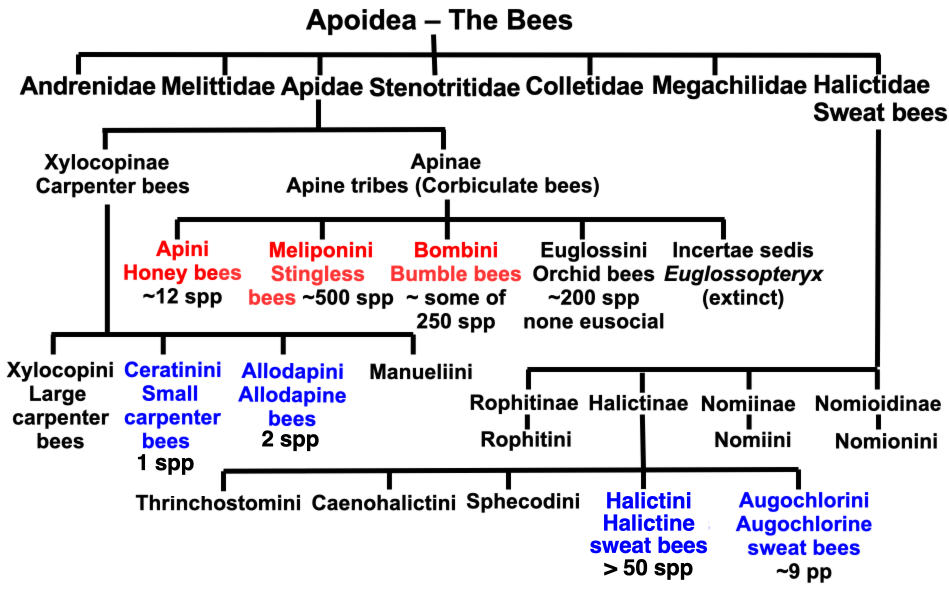
Figure 2.1 The bees showing the number of species that are eusocial:
Red = obligatory eusocial tribes; and
Blue = facultative eusocial tribes.
The Halictidae (sweat bees), like the Apidae (corbiculate and carpenter bees) originated in the late Cretaceous some 96-75 million years ago.vi Michener records colonies of Lasioglossum marginatum regularly reaching several hundred females in number in Europe, 342 females in a nest of Halictus lutescens in Costa Rica, a nest of over 589 females of the same species in Guatemala, and a nest of Halictus hesperus in Panama containing between 97 and 149 workers near the end of the nesting cycle. These sizes are, however, the exception. In exploring strategies bees adopt to build social nests researchers have rarely discovered a nest that contains more than 10-20 individuals. The zephyr sweat bee, Lasioglossum zephyrum (Figure 2.2), that forms nests with up to four female bees typifies this communally social bee group.vii Sometimes – as we shall discover – social behaviour is abandoned in favour of solitary existence. Now and then there is strong phylogenetic and genetic evidence of complete loss of any social facility.

Figure 2.2 Lasioglossum zephyrum, a communally nesting sweat bee.viii
The chart (Figure 2.3) provides an overview of just where advanced sociality has emerged amongst the bee families.
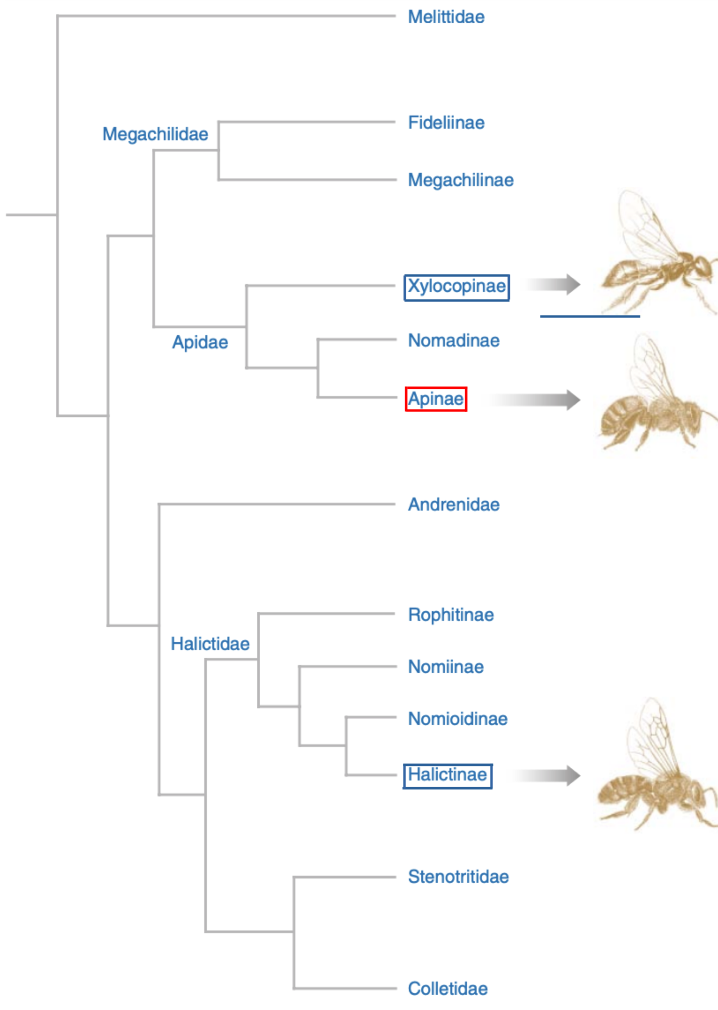
Figure 2.3 Phylogenetic associations of bee familiesix highlighting sub families with:
fully eusocial species (red); and
facultatively eusocial species (blue).
Family Halictidae The sweat bees
According to the University of Florida,x halictid sweat bees are overall – in terms of sheer numbers – the next most common bee encountered (4500 species) after the common honey bee. The majority of halictids construct nests by excavating branching tunnels in bare earth, others nesting in rotting wood. Cells are typically lined with a waxy exudate and, amongst the more social species, are often clustered.
The sweat bees (family Halictidae) comprise four sub families, three of which are purely solitary, the Rophitinae, the Nomiinae and the Nomioidinae. The remaining Halictinae sub family, however, has many facultatively eusocial species. Quoting Michener:xi
Halictids display the most diverse gradation in social behavior as species can be solitary, communal, semi-social or eusocial. Some species exhibit both solitary or eusocial behavior, termed facultatively eusociality.
In a broad scoping overview of the origin of social bees Bryan Danforthxii provides various clues as to the current diversity of halictid bees. He tells us that, within halictid sub family, eusociality arose three times and that reversion to solitary behaviour occurred at least twelve times. This leaves us with the Halictinae sub family with five tribes of which two tribes, the Halictini and Augochlorini, have all the extant eusocial taxa. Social (as opposed to eusocial) taxa exist elsewhere in the halictid bee family, so we will also look briefly at one other sub family, the Nomiinae and its single tribe Nomiini that has many social genera.
Sub family Halictinae
Thus of the five tribes of the bee sub family Halictinae, only the Halictini and Augochlorini tribes have eusocial taxa. Of these species, Halictus and Lasioglossum (Halictini) and Augochlora and Augochlorella (Augochlorini) comprise the bulk of known non corbiculate eusocial bees. Confusingly some subgenera, for example of Lasioglossum, are sometimes assigned genus status signalling the ever present efforts of taxonomists to reclassify the names of taxa that we mere mortals thought were fixed for all time.
Tribe Halictini Halictine sweat bees
The tribe Halictini has twenty five genera, the best known and with the most species being Lasioglossum (1800 species worldwide with ~ 250 Australian species) noted for their behavioural plasticity and the closely related Halictus genus (about 200 species). Richards and Packer observe that of the roughly 1800 Lasioglossum species, about 112 have been well studied. Of these forty two species have been described as solitary, about thirty eusocial, eleven communal and one other semi social. Of the genus Halictus they indicate that the reproductive behaviour of about twenty eight species have been investigated. Of these twenty are eusocial.
The diversity of social expression in the Halictinae (Halictini + Augochlorini) is highlighted by Eickwort and Kukuk:xiii
Primitively eusocial behavior has evolved in nine lineages of the Halictinae (Halictus, Seladonia, Evylaeus, Dialictus, Augochlora, Augochlorella, Pereirapis and one species each of Lasioglossum s.s and an African subgenus of Lasioglossum s.I). All but two of these lineages also contain solitary species, so reproductive castes have evolved independently and/or been lost multiple times in the sweat bees.
Two basic patterns of social organization are apparent in the Halictinae. In the more advanced pattern exemplified by Evylaeus malachrus workers and reproductives are produced in discrete broods, castes are typically distinct, and queens live through the life of the colony. In the more primitive pattern exemplified by Dialictus zephyrus and Dialictus lineatulus there is no separation between the production of worker and reproductive broods, queen and worker castes overlap broadly in size and physiology, the queens frequently die before the production of reproductives in the nest and are replaced by sisters of the workers.
Eickwort describes the atypical life system system of a primitively eusocial halictine bee, Lasioglossum (Dialictus) lineatulus [syn Dialictus lineatulum],xiv where a range of one to six queens establish a common nest. By summer there are up to an average of seven queens in the nest, one that does not forage remaining in the rest together with daughter workers.. At this stage the colony is fully eusocial. However the larger foundress queen fairly soon dies and in this condition the colony becomes semi social, that is functionally queenless. The older daughters of the queen then take over the nest forcing younger daughters of the originally subordinate queen into a worker role. It appears that reproductive castes prevent subordinate females establishing their own nests and this may represent an early stage of evolution of a more defined worker caste of bees. Others such as Kocher and coworkersxv have employed genetic tools to track the evolution of halictid eusociality.
Miriam Richards study of Halictus ligatus in southern Ontario,xvi under variable inter annual seasonal conditions, is revealing:
Under the harsher weather conditions of 1990, mortality was higher, colony sizes were lower, brood body sizes were smaller, and surviving colonies were more strongly eusocial than under the milder conditions of 1991.
In another instructive study Soucy and Danforthxvii (and independently Yanegaxviii) report on the behaviour of the bee Halictus rubicudus, a eusocial species that migrated across the Pleistocene land bridges from Eurasia to North America. The species adapted to the the Rocky Mountains climate, where the season is short, by reverting to a purely solitary condition. Quoting various sources, Laurence Packerxix instances other halictine species exhibiting this divergent social behaviour: Lasioglossum calceatum in France is solitary at high elevation and eusocial at low elevation, a finding replicated in a study by Davison and Field in a similar study of same species in Japanxx. Halictus ligatus in Florida, is similarly sensitive changing its normally eusocial behaviour to solitary nesting under unseasonably cold conditions.xxi In a further study Wyman and Richards studied the nesting behaviour Lasioglossum malachurum in southern Greece.xxii They discovered that not only did this bee go through more generations in this warmer climate, but that the first two generations comprised workers only, whereas the third generation contained a mix of workers, drones and gynes, the latter incipient female reproductives.
Gibbs, Brady, Kanda and Danforthxxiii have proposed a common progenitor of eusociality in the large Halictini genera Halictus and Lasioglossum. Danforth, Conway and Jixxiv have also signalled multiple losses of eusociality within this primitively eusocial bee clade. Having explored the social behavioural response of the Lasioglossum–Halictus group to conditions we now need to delve more into their social dynamic.xxv
The Australian Lasioglossum hemichalceum is a communal species (Figure 2.4).xxvi Individual queens, unrelated to each other, cooperate and live in the same nest and 1-8 offspring are produced per queen. With eight Australian subgenera, and many species, additional endemic species of Lasioglossum may well turn out to be social or even eusocial.

Figure 2.4 Australian social halictine bee Lasioglossum hemichalceum.xxvii
Kukuk discusses the downside dynamics of social communities where there is a risk of a queen having her eggs cannibalised and replaced by a nest mate. Conversely there are advantages accrued to a colony when an adult non forager remains to guard the nest or where a queen that forages is lost and her brood can be nurtured by a surrogate female.
One exemplar of facultative eusociality is found in Lasioglossum albipes spread right across the Palearctic region (much of the northern hemisphere)where it exists either as a solitary or primitively eusocial bee. Kocher and coworkersxxviii note that even with one generation per year that:
…this socially polymorphic species is solitary in inland localities in France and Germany, but eusocial in southwestern France where the climate is warmer and nests are initiated earlier in the summer.
Eusociality is so common in the Lasioglossum genus, that Danforth, Conway and Jixxix have mapped out their interrelatedness spanning solitary, sub social and eusocial species.
Some species are not just communal (two or more females living in the same nest) but are facultatively eusocial. With Halictus sexcinctus the level of sociality varies from communal (simple nest sharing) through to intergenerational colony development where worker castes contribute to provisioning and nurturing queen progeny (a eusocial nest condition). In warmer southern Greecexxx this species is bivoltine (two generations per year). Instead of being solitary Halictus sexcinctus becomes weakly eusocial.
Tribe Augochlorini Augochlorine sweat bees
The social biology of the augochlorine sweat bees has been studied by Dalmazzo and Roig-Alsina,xxxi while their evolutionary biology has been evaluated evaluated by Danforth and Eickwort.xxxii Three genera, Augochlorella, Pereirapis and Augochlora (Oxystoglossella), contain primitively eusocial taxa, Danforth and Eickwort’s listing nine such species. Inter alia they reference Lawrence Packer’s study of Augochlorella striata where a mixture of solitary and eusocial populations exist right to at the northern end of its range at Cape Breton Island in Nova Scotia.xxxiii In socially flexible sweat bees such as Augochlorella aurata, nest size and architecture reflects the level of social organisation (Figure 2.5).
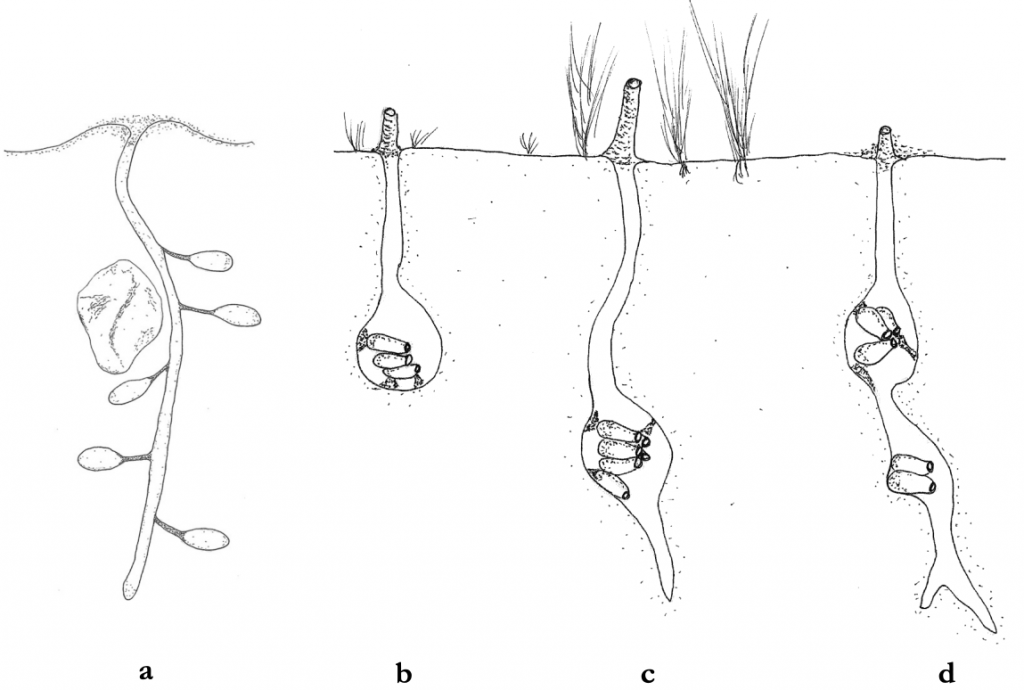
Figure 2.5 Nesting arrangments of solitary and facultatively eusocial halictine bees:xxxiv
(a) nest of solitary Lasioglossum leucozonium that has one generation per year and that constructs the nest alone; and
Augochlorella aurata with:
(b) a solitary nest of a single foundress:
(c) an extended solitary nest; and
(d) a eusocial nest with a foundress producing second brood with worker support.
Eickwort and Sakagami signal wide variability in nest architecture across many genera in the Augochlorini tribe (Figure 2.6).xxxv
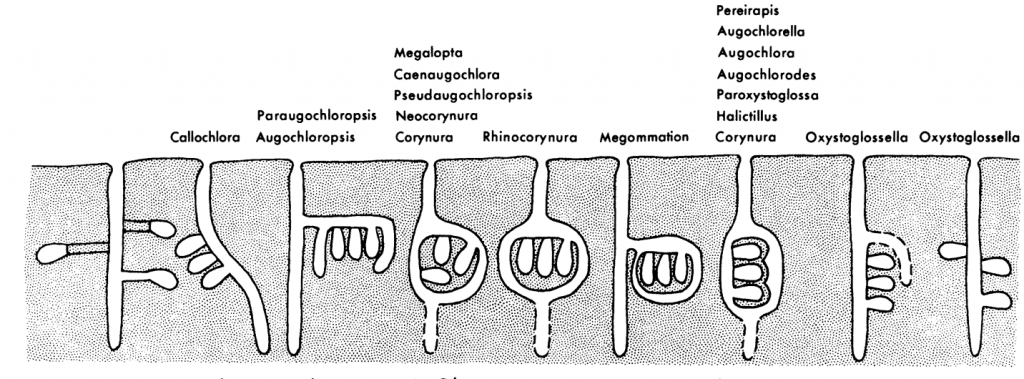
Figure 2.6 Nest structures in the tribe Augochlorini.
Eickwort and Sakagami also describe the nest structure of a Brazilian Augochlorini sweat bee, Rhinocorynura inflaticeps, where cells are aggregated as a comb at the end of a lateral tunnel (Figure 2.7). Notably the cells are formed as individual cups and not consolidated as we find in honey bees and some vespine wasps.
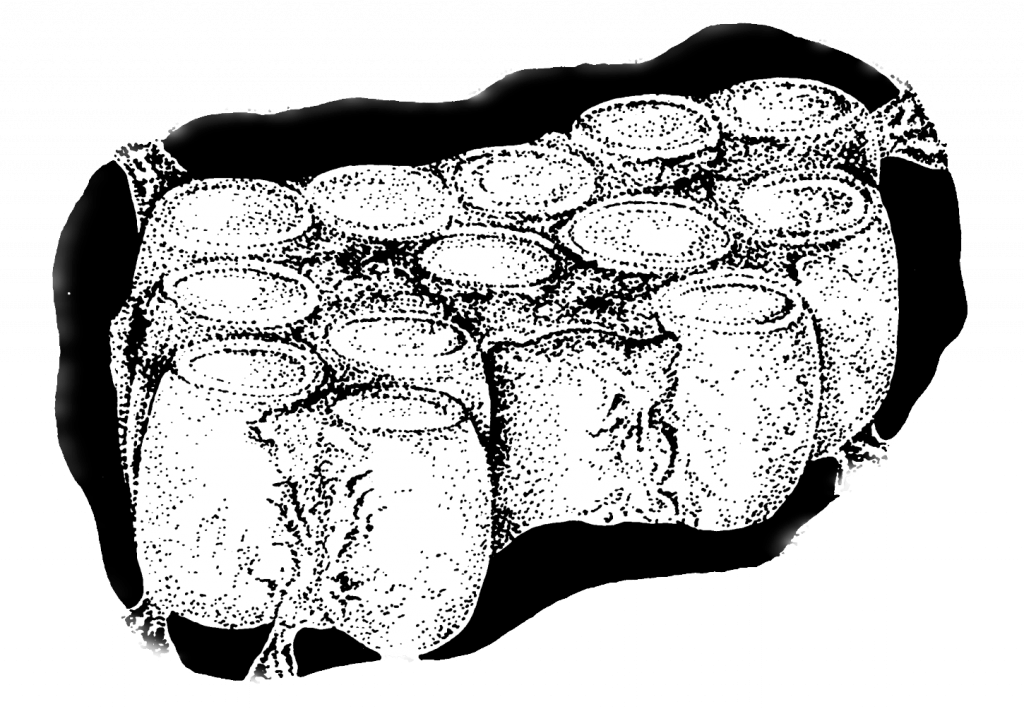
Figure 2.7 Polymorphic Brazilian augochlorine bee Rhinocorynura inflaticeps nest in a single blind tunnel.xxxvi
Sub family Nomiinae
The sub family Nomiinae has a single tribe, Nomiini that contains a few highly gregarious, but not eusocial species. Their nest structures are of some note and signal a high level of cooperation, despite the absence of a worker caste. The sub family Nomiinae, nomia bees, are common in Australia and comprise about 25% of Australian native bee species, all of which are essentially solitary. After honey bees they are extremely commonly encountered bees.xxxvii
Tribe Nomiini Nomiine (Nomia) bees
Wcislo and Engel have reviewed the sparse literature on the nomia bees and concluded that a large proportion of species display some measure of sociality, multiple queens often sharing a common burrow. Typically species in several genera (e.g. Nomia, Austronomia, Pseudoapis and Afronomia) build nests on flat ground and share a common funnel entrance, individual queens guarding the nest and positing an egg with pollen and nectar in teardrop shaped cells in side tunnels (Figure 2.8). Cells are lined with a water repellant secretion produced by Dufour’s glands.
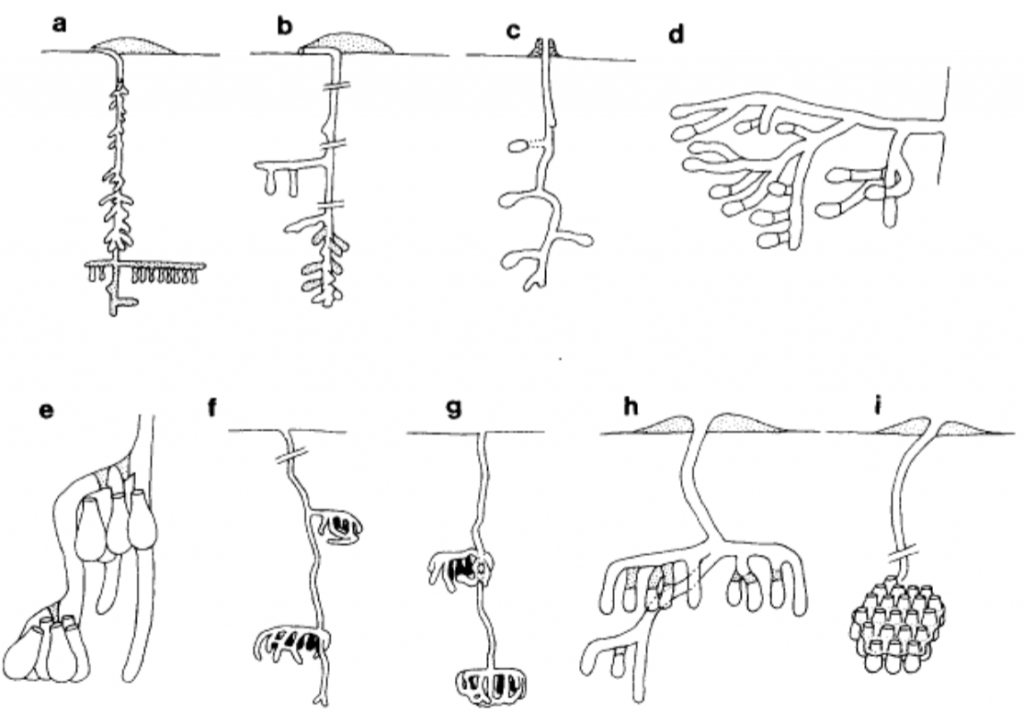
Figure 2.8 Nest structures of social Nomiinae halictid bees:xxxviii
(a) Dienunomia triangulifera;
(b) Dieunomia heteropoda;
(c) Rhopalomella esakii;
(d) Afronomia sjostedti;
(e) Lipotriches australica;
(f) Nomia capitata;
(g) Nomia oxybeloides;
(h) Holonomia pulchribalteata; and
(i) Nomia unidentata.
An interesting example of nesting behaviour is found in Lipotriches (Austronomia) australica (Figure 2.9). Nests in southwestern Victoriaxxxix have been found to have one, two and three queens, each colony member sharing duties such as foraging. They fully provision a single cell and seal it, before moving on to excavate and construct a new cell. In an earlier report Kukukxl notes that nest mates of Austronomia australica were less antagonistic towards pairs of females from the same nest than from different nests. To the extent that individual queens are not subordinate to any dominant queen and there is no worker caste, nomia bees are not eusocial. Nevertheless the level of social organisation is remarkable.

Figure 2.9 Australian nomiine bee Lipotriches (Austronomia) australica.xli
Readings
iEncyclopedia Britannica (accessed 25 June 2023). Spontaneous generation biological theory. https://www.britannica.com/science/spontaneous-generation
iiSchwarz, M.P., Richards, M.H. and Danforth, B.N. (2007). Changing paradigms in insect social evolution: New insights from halictine and allodapine bees. Annual Review of Entomology 52(1):127-150. doi:10.1146/annurev.ento.51.110104.150950
iiiMichener, C.D. (1990). Reproduction and castes in social halictine bees, Chapter 4. pp.77-121. In Engels, W. (ed) Social insects: An evolutionary approach to castes and reproduction. Springer, Berlin, Heidelberg.
doi:10.1007/978-3-642-74490-7_6
ivMichener, C.D. (1974). The social behavior of the bees: A comparative study. Harvard University Press. https://archive.org/details/socialbehaviorof0000mich/page/n6/mode/1up
vMichener, C.D. (1990). Castes in xylocopine bees, Chapter 5. pp.123-146. In Engels, W. (ed) Social insects: An evolutionary approach to castes and reproduction. Springer, Berlin, Heidelberg. doi:10.1007/978-3-642-74490-7_7
viGibbs, J., Brady, S.G., Kanda, K. and Danforth, B.N. (2012). Phylogeny of halictine bees supports a shared origin of eusociality for Halictus and Lasioglossum (Apoidea: Anthophila: Halictidae). Molecular Phylogenetics and Evolution 65(3):926-939. doi:10.1016/j.ympev.2012.08.013
viiBatra, S.W. (1964). Behavior of the social bee, Lasioglossum zephyrum, within the nest (Hymenoptera: Halictidae). Insectes Sociaux 11(2):159-185. https://www.researchgate.net/profile/Suzanne-Batra/publication/332413354_Behavior_of_the_social_bee_Lasioglossum_zephyrum_within_the_nest_Hymenoptera_Halictidae/links/5cb4079592851c8d22ec40b3/Behavior-of-the-social-bee-Lasioglossum-zephyrum-within-the-nest-Hymenoptera-Halictidae.pdf
viiiiNaturalist (accessed 8 October 2023). Zephreus sweat bee. https://www.inaturalist.org/photos/37979962
ixSchwarz, Richards and Danforth. (2007) loc. cit.
xBuckley, K., Nalen, C.Z. and Ellis, J. (2011). Hallictid bees. University of Florida. https://entnemdept.ufl.edu/creatures/misc/bees/halictid_bees.htm
xiMichener, C.D. (2007). The Bees of the World, second edition. The Johns Hopkins University Press, Baltimore, Maryland. Solitary versus social life. Section 5. pp.12-15.
xiiDanforth, B.N. (2002). Evolution of sociality in a primitively eusocial lineage of bees. Proceedings of the National Academy of Sciences of the United States of America 99(1):286-290. doi:10.1073/pnas.012387999
xiiiEickwort, G.C. and Kukuk, P.F. (1987). Reproductive castes in primitively eusocial halictid bees. In Eder, J. and Rembold, H. (eds). Chemistry and biology of social insects. Peperny, Münich, pp.261-262. https://archive.org/details/chemistrybiology0000unse_m3l8/page/261/mode/1up
xivEickwort, G.C. (1986). First steps into eusociality: The sweat bee Dialictus lineatulus. The Florida Entomologist 69(4):742-754. https://www.jstor.org/stable/3495222?read-now=1#page_scan_tab_contents
xvKocher, S.D., Mallarino, R., Rubin, B.E.R., Yu, D.W., Hoekstra, H.E. and Pierce, N.E. (2018). The genetic basis of a social polymorphism in halictid bees. Nature Communication 9(1):4338. doi:10.1038/s41467-018-06824-8 https://pubmed.ncbi.nlm.nih.gov/30337532/
xviRichards, M.H. (2004). Annual and social variation in foraging effort of the obligately eusocial sweat bee, Halictus ligatus (Hymenoptera: Halictidae). Journal of the Kansas Entomological Society 77(4):484-502. https://www.researchgate.net/profile/Miriam-Richards/publication/232686660_Annual_and_Social_Variation_in_Foraging_Effort_of_the_Obligately_Eusocial_Sweat_Bee_Halictus_ligatus_Hymenoptera_Halictidae/links/5e0a3614a6fdcc28374acbc0/Annual-and-Social-Variation-in-Foraging-Effort-of-the-Obligately-Eusocial-Sweat-Bee-Halictus-ligatus-Hymenoptera-Halictidae.pdf
xviiSoucy, S.L. and Danforth, B.N. (2002). Phylogeography of the socially polymorphic sweat bee Halictus rubicundus (Hymenoptera: Halictidae). Evolution 56(2):330-341. doi:10.1111/j.0014-3820.2002.tb01343.x
xviiiYanega, D. (1988). Social plasticity and early-diapausing females in a primitively social bee. Proceedings of the National Academy of Sciences of the United States of America 85(12):4374-4377. doi:10.1073/pnas.85.12.4374
Yanega, D. (1989). Caste determination and differential diapause within the first brood of Halictus rubicundus in New York (Hymenoptera: Halictidae). Behavioral Ecology and Sociobiology 24(2):97-107. doi:10.1007/BF00299641
Yanega, D. (1992). Does mating determine caste in sweat bees (Hymenoptera, Halictidae)? Journal of the Kansas Entomological Society 65(3):231-237. www.jstor.org/stable/25085361
Yanega, D. (1993). Environmental influences on male production and social structure in Halictus rubicundus (Hymenoptera: Halictidae). Insectes Sociaux 40(2):169-180. doi.org/10.1007/BF01240705
Yanega, D. (1997). Demography and sociality in halictine bees (Hymenoptera: Halictidae). Chapter 14. pp.293-315 In Choe, J.C. and Crespi, B.J. (eds). The evolution of social behavior in insects and arachnids. Cambridge University Press, Cambridge, U.K. https://sci-hub.mksa.top/10.1017/CBO9780511721953.015
doi:10.1017/CBO9780511721953.015
xixPacker, L. (1990). Solitary and eusocial nests in a population of Augochlorella striata (Provancher) (Hymenoptera; Halictidae) at the northern edge of its range. Behavioral Ecology and Sociobiology 27(5):339-344. doi:10.1007/BF00164004
xxDavison, P.J. and Field, J. (2018). Limited social plasticity in the socially polymorphic sweat bee Lasioglossum calceatum. Behavioral Ecology and Sociobiology 72(3):1-13. https://link.springer.com/article/10.1007/s00265-018-2475-9 https://www.jstor.org/stable/44857152
xxiPacker, L. and Knerer, G. (1986). The biology of a subtropical population of Halictus ligatus Say (Hymenoptera: Halictidae). I. Phenology and social organisation. Behavioral Ecology and Sociobiology 18(5):363-375. https://www.jstor.org/stable/4599902
Packer, L. (1986). The biology of a subtropical population of Halictus ligatus Say (Hymenoptera; Halictidae). II. Male behaviour. Ethology 72(4):287-298. doi:10.1111/j.1439-0310.1986.tb00630.x
Packer, L. and Knerer, G. (1987). The biology of a subtropical population of Halictus ligatus Say (Hymenoptera; Halictidae). III. The transition between annual and continuously brooded colony cycles. Journal of the Kansas Entomological Society 60(4):510-516. https://www.yorku.ca/bugsrus/resources/publications/1987%20Packer%20and%20Knerer%20[].pdf
Carman, G.M. and Packer, L. (1996). A cryptic species allied to Halictus ligatus Say (Hymenoptera: Halictidae) detected by allozyme electrophoresis. Journal of the Kansas Entomological Society 69(4):168-176. http://www.jstor.org/stable/25085714
xxiiWyman, L.M. and Richards, M.H. (2003). Colony social organization of Lasioglossum malachurum Kirby (Hymenoptera, Halictidae) in southern Greece. Insectes Sociaux 50(3):201-211. doi:10.1007/s00040-003-0647-7 https://link.springer.com/article/10.1007/s00040-003-0647-7
Wikipedia (accessed 25 June 2023). Lasioglossum malachurum. https://en.wikipedia.org/wiki/Lasioglossum_malachurum
xxiiiGibbs, Brady, Kanda and Danforth (2012) loc. cit.
xxivDanforth, B.N., Conway, L. and Ji, S. (2003). Phylogeny of eusocial Lasioglossum reveals multiple losses of eusociality within a primitively eusocial clade of bees (Hymenoptera: Halictidae). Systematic Biology 52(1):23-36. doi.org/10.1080/10635150390132687
Jones, B.M., Rubin, B.E.R., Dudchenko, O., Kingwell, C.J., Traniello, I.M., Wang, Z.Y., Kapheim, K.M., Wyman, E.S., Adastra, P.A., Liu, W., Parsons, L.R., Jackson, S.R., Goodwin, K., Davidson, S.M., McBride, M.J., Webb, A.E., Omufwoko, K.S., Van Dorp, N., Otárola, M.F., Pham, M., Omer, A.D., Weisz, D., Schraiber, J., Villanea, F., Wcislo, W.T., Paxton, R.J., Hunt, B.G., Aiden, E.L. and Kocher, S.D. (2023). Convergent and complementary selection shaped gains and losses of eusociality in sweat bees. Nature Ecology and Evolution 7(4):557-569. https://www.nature.com/articles/s41559-023-02001-3
xxvDar, S.A., Javeed, K., Al-Shuraym, L.A., Sayed, S., Devi, Y.K., Hassan, W., Ahmed, M.M.M., Alkenani, N.A., Gajger, I.T. and John, J. (May 2023). Assessment of nest architecture and pollination efficiency of Lasioglossum (Evylaeus) marginatum (Halictidae: Hymenoptera). Biologia 78(5):1-13.
https://link.springer.com/article/10.1007/s11756-023-01419-1#citeas
https://www.researchgate.net/profile/Showket-Dar/publication/370709912_58_Assessment_of_nest_architecture_and_pollination_efciency/links/645e53c3434e26474fe109f5/58-Assessment-of-nest-architecture-and-pollination-efciency.pdf
xxviKukuk, P.F., Forbes, S.H.R., Zahorchack, R., Riddle, A. and Pilgrim, K. (2002). Highly polymorphic microsatellite markers developed for the social halictine bee Lasioglossum (Chilalictus) hemichalceum. Molecular Ecology Notes 2(4):529-530. https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=6913753b6e7fedcd27d0d8f78a2a91c80e5918db
Kukuk, P.F. and Sage, G.K. (1994). Reproductivity and relatedness in a communal halictine bee Lasioglossum (Chilalictus) hemichalceum. Insectes Sociaux 41(4):443-455. doi:10.1007/BF01240647
Kukuk P.F., Ward, S.A. and Jozwiak, J. (1998). Mutualistic benefits generate an unequal distribution of risky activities among unrelated group members. Naturwissenschaften 85(9):445-449. doi:10.1007/s001140050528 https://link.springer.com/article/10.1007/s001140050528
xxviiiNaturalist (accessed 16 October 2023). Photos of Lasiglossum hemichalceum. ahttps://www.inaturalist.org/taxa/892286-Lasioglossum-hemichalceum/browse_photos
xxviiiKocher, S.D., Li, C., Yang, W., Tan, H., Yi, S.V., Yang, X., Hoekstra, H.E., Zhang, G., Pierce, N.E. and Yu, D.W. (2013). The draft genome of a socially polymorphic halictid bee, Lasioglossum albipes. Genome Biology 14(12):R142. doi:10.1186/gb-2013-14-12-r142
xxixDanforth, Conway and Ji (2003) loc. cit.
xxxRichards, M.H. (2001). Nesting biology and social organization of Halictus sexcinctus (Fabricius) in southern Greece. Canadian Journal of Zoology 79(12):2210-2220. https://www.researchgate.net/publication/237402258_Nesting_biology_and_social_organization_of_Halictus_sexcinctus_Fabricius_in_southern_Greece
xxxiDalmazzo, M. and Roig-Alsina, A. (2015). Social biology of Augochlora (Augochlora) phoemone (Hymenoptera: Halictidae) reared in laboratory nests. Insectes Sociaux 62(3):315-323.
doi:10.1007/s00040-015-0412-8 https://link.springer.com/article/10.1007/s00040-015-0412-8
xxxiiDanforth, B.N. and Eickwort, G.C. (1997). The evolution of social behavior in the augochlorine sweat bees (Hymenoptera: Halictidae) based on a phylogenetic analysis of the genera, Chapter 13, pp.270-292. In Choe, J.C. and Crespi, J.C. 2010 (eds). The evolution of social behaviour in insects and arachnids, Cambridge University Press. doi:10.1017/CBO9780511721953.014
xxxiiiPacker (1990) loc. cit.
xxxivRichards, M.H. and Packer, L. (2020). Sweat bees (Halictidae) pp.934-942; Packer, L. and Richards, M.H. Halictus, pp.473-476; Richards, M.H. and Packer, L Lasioglossum pp.550-554. In Starr, C. (ed). Encyclopedia of Social Insects. Springer, Cham. doi:10.1007/978-3-030-28102-1_11 https://sci-hub.mksa.top/10.1007/978-3-030-28102-1_117 https://link.springer.com/referenceworkentry/10.1007/978-3-319-90306-4_124-1
xxxvEickwort, G.C. and Sakagami, S.F. (1979). A classification of nest architecture of bees in the tribe Augochlorini (Hymenoptera: Halictidae; Halictinae), with description of a Brazilian nest of Rhinocorynura inflaticeps. Biotropica 11(1):28-37. https://www.jstor.org/stable/2388168
xxxviEickwort and Sakagami (1979) loc. cit.
xxxviiAustralian Museum (accessed 26 May 2023). Nomia bees. https://australian.museum/learn/animals/insects/nomia-bees/
xxxviiiWcislo, W.T. and Engel, M.S. (1996). Social behavior and nest architecture of Nomiine bees (Hymenoptera: Halictidae; Nomiinae). Journal of the Kansas Entomological Society 69(4):158-167. Supplement: Special Publication Number 2: Proceedings of the Eickwort Memorial Symposium. https://www.jstor.org/stable/25085713
xxxixVogel, M.E. and Kukuk, P.F. (1994). Individual foraging effort in the facultatively social halictid bee, Nomia (Austronomia) australica (Smith). Journal of the Kansas Entomological Society 67(3):225-235. https://www.jstor.org/stable/2508551VVV
xlKukuk, P.F. (1990). Nestmate recognition in a group-living nomiine bee (Halictidae, Nomiinae). In Veeresh, G.K., Mallik, B. and Viraktamath, C.A. (eds). Social Insects and The Environment. pp.509-510. https://archive.org/details/socialinsectsenv0000inte/page/509/mode/1up
xliAtlas of Living Australia (accessed 16 October 2023). Lipotriches (Austronomia) australica (Smith, 1875) https://bie.ala.org.au/species/https://biodiversity.org.au/afd/taxa/1a2d7242-f1d6-46d4-925a-a7ac5d9a2434#gallery
