
Alan Wade
How are the mighty fallen,
and the weapons of war perished!
2 Samuel 1:27 KJV
Canada Bill [1847-1887], born in Yorkshire William Jones, migrated to North America first working on the Mississippi River as a card sharp, then later in the gambling dens on the Union Pacific Railroad. Had anyone asked Canada Bill how to banish Varroa mites he would have introduced himself gawkishly, proffered a very plausible and foolproof solution and sought an upfront payment, a bargain for such considered advice.
Canada Bill was a confidence trickster par excellence. He invariably walked away on the best of terms and with a well-lined pocket. He reminds us eerily of a similarly inclined prime minister who instead migrated to Australia. Much to the merriment of the hoi polloi he professed to know all about ‘Canadia’.
As we are now discovering, mites and their virus partners in crime are tricky creatures and are not so readily misled. With many assurances that mites could be fully controlled with synthetic miticides beekeepers have everywhere discovered that the mites actually knew better. They, the mites, soon adapted and very quickly got into bed with the viruses with a pact to make the situation eventually worse for bees and beekeepers alike.
Natural defences of other honey bees
But what solutions might honey bees provide? What can they tell us about the defences they have put up in the course of evolution and are continuing to learn today?
Destructive mites and their apine hosts come in many colours. First and foremost we learn that the original host bees and their parasites have learnt to live with each other. The mites, like Canada Bill, extracted their wages of sin but did not kill their victims (Table 1).
| Order/Clade | Family | Parasite species | Likely primary hosts |
| Sarcoptiformes/Astigmatina | Tarsonemidae | Acarapis woodi [Tracheal mite] | Apis mellifera |
| Acarapis externus Acarapis dorsalis [External mites]412 | Not known but found on Apis dorsata, Apis cerana and Apis mellifera in Asia | ||
Parasitiformes/Mesostigmata | Varroidaei | Varroa destructor [Varroa mite] | Apis cerana |
| Varroa jacobsoni Varroa rindereri Varroa underwoodi | Likely Apis cerana Apis koschevnikovi Apis cerana | ||
| Euvarroa sinhai Euvarroa wongsirii | Apis florea Apis andreniformis | ||
| Laelapidaeii | Tropilaelaps clareae Tropilaelaps mercedesae Tropilaelaps koenigerum Tropilaelaps thaii | Apis breviligula Apis dorsata Apis dorsata Apis laboriosa | |
| Trombidiformes/Prostigmata | Erythraeidae | Leptus ariel Leptus monteithi? Leptus spp | Apis mellifera |
Table 1 Relationships of predatory mites to their natural honey bee hosts..
Of these mites a few, notably Varroa destructor, Varroa jacobsoni, Tropilaelaps mercedesae and Tropilaelaps clareae have jumped to the European honey bee with devastating consequence. The sudden appearance of Acarapis woodi as a parasite of Apis mellifera early in the 20th Century remains a mystery.
Others mites such as Euvarroa sinhai have been raised in Apis mellifera colonies, those juxtaposed to Apis florea colonies, but have not become debilitating parasites. Still others have migrated to other honey bees (e.g. the same Euvarroa sinhai from Apis florea to Apis andreniformis and Tropilaelaps mercedesae from Apis dorsata to its giant honey bee relatives) but that is another story.
Hope for our honey bees?
More perplexing has been the discovery of small ‘island populations’ of Varroa resistant Apis mellifera. These honey bees have successfully selected themselves or have been selected to live with Varroa (Table 2). As a rule of thumb, these bees have not retained their Varroa tolerance when translocated. Efforts to fix such a desirable trait has been confounded by outbreeding, aka open mating, that occurs when bees swarm or where a colony supersedes its queen.
Further developing resistance of mites to treatment chemicals is providing cover for viruses whose natural virulence is masked. Viruses vectored by Varroa destructor include not only Deformed Wing Virus but also Israeli Acute Bee Paralysis Virus, Sacbrood Virus, Kashmir Bee Virus, Black Queen Cell Virus and Acute Bee Paralysis Virus. Tropilaelaps mercedesae similarly vectors Deformed Wing Virus and Black Cell Queen Virus though its capacity to host other viruses is yet to be established.
| Country | Region |
| Africaiii | South Africa, various other regions in Africa |
| Brazil and Paraguayiv | Appears to be country wide amongst Apis mellifera scutellata hybrid colonies |
| Francev | Avignon and Le Mans, France |
| Germanyvi | Isolated forests |
| North Americavii | Arnot Forest, NY State |
| Norwayviii | Østlandet region |
| Papua New Guinea/Solomon Islandsix | Papua New Guinea highlands |
| Russiax | Primorsky region, far eastern Russia |
| Swedenxi | Island of Gotland |
| The Netherlandsxii | Tiengemeten and Amsterdamse Waterleidingduinen (in part of Gotland origin) |
Table 2 Honey bee bioregions with Varroa tolerance. Interestingly Africanised bees in Mexico with scutellata genetics have typically low tolerance to Varroa mites.xiii
Overall the picture of mite impact on honey bees is far from complete though a wide-ranging survey of mite origins and their spread by Sammataro and coworkersxiv provides an excellent retrospective on the still emerging mite problem. Their survey also covers a range of commensal, scavenger and pollen consuming mites that either do little damage, are beneficial in removing fungal debris, or are phoretic flower mites whose presence in bee colonies is incidental (Table 3).
| Order | Species | Bee-mite association |
| Astigmata | Forcellinia faini | Scavenger of bee and insect debris and fungi |
| Prostigmata | Pseudacarapis indoapis | Pollen feeder in Apis cerana colonies |
| Mesostigmata | Melichares dentriticus | Predator of scavenger mites |
| Melittiphis alvearius | Globally distributed pollen feeding mite in Apis mellifera colonies | |
| Afrocypholaelaps africana | Flower mites phoretic on honey bees | |
| Afrocypholaelaps spp | Flower mite | |
| Hypoaspis spp | Predatory mainly soil mites | |
| Melichares dentriticus | Predator of scavenger mites | |
| Melittiphis alvearius | Pollen feeder | |
| Neocypholaelaps spp | Flower mites phoretic on honey bees |
Table 3 Common non-parasitic mites in honey bee (Apis mellifera) colonies.
They argue that widespread external honey bee mites in the Acarapis genus are of uncertain origin and that the genesis of the Isle of Wight outbreak, widely attributed to Acarapis woodi, remains contentious. The existence of two relatively innocuous external mites Acarapis externus and Acarapis dorsalis in Asian honey bees suggests a source of the genus lies in much deeper time. The tracheal mite Acarapis woodi, though recently causing high colony mortality in Asian and Asian populations of introduced western honey beesxv, is now much less of a long term threat. In Europe bees have either acquired some immunity or that the mite (and likely its viruses) are far less lethal than they were a century ago.
Migration of mites between honey bee species and their impacts is much more common than has been commonly supposed, the severe impact of the tracheal mite on the farmed Apis cerana japonica being a recent prime example (Table 4).
| Mite species | Original hosts | Other Apis hosts |
| Acarapis dorsata Acarapis externus | Not known | Apis dorsata, Apis cerana, Apis mellifera |
| Acarapis woodi | Apis mellifera (?) | Apis cerana japonica |
| Euvarroa sinhai | Apis floreaxvi | Apis andreniformis, Apis mellifera |
| Euvarroa wongsirii | Apis andreniformis | |
| Leptus ariel, Leptus monteith?i*, | Various unknown arthropodsxvii | Apis mellifera |
| Tropilaelaps clareae | Apis dorsata binghami Apis breviligula | Apis mellifera Apis mellifera, Apis cerana |
| Tropilaelaps koenigerum | Apis dorsata, Apis laboriosa | |
| Tropilaelaps mercedesae | Apis dorsata, Apis laboriosaxviii | Apis mellifera, Apis mellifera scutellata, Apis cerana, Apis indica, Apis florea, Xylocopa iridipennis |
| Tropilaelaps thaii | Apis laboriosa | |
| Varroa destructor | Apis cerana | Apis mellifera |
| Varroa jacobsoni | Apis cerana | Apis mellifera, Apis koschevnikovi, Apis nuluensis |
| Varroa rindereri | Apis koschevnikovi | Apis cerana, Apis nuluensis |
| Varroa underwoodi | Apis cerana | Apis nigrocinta, Apis nuluensis |
* Associated with Spiroplasma bacteria the cause of May disease
Table 4 Acari mite migration to new bee hosts.
The devastating impact of Varroa destructor on the Western Honey Bee remains complex and only partially resolved.
The threat posed by Tropilaelaps (tropi) mites is arguably greater than that inflicted by the Varroa parasite. To quote the well-regarded Samuel Ramsey: Tropilaelaps is ‘A fate far greater than Varroa.’ Two species, Tropilaelaps mercedesae and Tropilaelaps clareae, are of particular concern. As noted elsewhere: xix, xx, the widely distributed Tropilaelaps mercedesae is found across mainland Asia… [including] Papua New Guinea where it is found on Apis mellifera despite this country being free of giant honey bees.
Denis Anderson and Matthew Morgan state that:xxi
Tropilaelaps clareae appears to be much more restricted being found only in the Sulawesi in Indonesia and the Phillipines outside the Palawan Islands. Tropilaelaps mercedesae, with which Tropilaelaps clareae has been confused, is considered to be the mite most threatening to the global beekeeping industry.
Chantawannakul, Ramsey, vanEngelsdorp, Khongphinitbunjong, and Phokasemxxii report the contemporary distribution of tropi mites associated with Apis mellifera:
- Tropilaelaps mercedesae in Myanmar, Thailand, Laos, Malaysia, Vietnam, Indonesia, the Philippines (Palawan Islands), South Korea, Hong Kong, Taiwan, China, Nepal, Bhutan, India, Sri Lanka, Iran, Afghanistan, Pakistan, Kenya, and Papua New Guinea; and
- Tropilaelaps clareae in the Philippines and the Sulawesi where its original hosts are the Philippine Giant Honey bee Apis breviligula (i.e. excluding the Palawan Islands where the very widespread Apis dorsata is instead present) and the Sulawesi Giant Honey Bee Apis dorsata binghami.
These authors signal that the current principal threat is with Tropilaelaps mercedesae. It has been found in other Asian honey bees, Apis cerana in Thailand, Pakistan, and Myanmar, Apis florea and Apis indica in India; Apis laboriosa in Vietnam and Apis dorsata in the Palawan Islands in the Philippines. However Apis dorsata and Apis cerana have inherent defences against Tropilaelaps infestations: Apis dorsata recognises and imprisons mites in sealed brood cells and also migrates seasonally when mites do not survive, while Apis cerana shakes and grooms off mites.
Stuart Robert reports the recent finding of Tropilaelaps mercedesae mite (further north than Iran, Afghanistan and Pakistan) in southern Russia and Uzbekistanxxiii signalling that it may well spread to and devastate beekeeping in Europe, his colleague noting that:
Considering that Varroa also went to Europe through our country [Russia] and that the rate of spread of Tropilaelaps may be faster, it will probably reach Europe within 3-5 years. It is not quite clear how it over-winters, given its biology, but it manages to do so.
Chantawannakul and coworkersxxiv also note that tropi mites can only survive in broodless colonies for seven days or no longer than three days with caged bees. These workers also discuss other important dynamics of Tropilaelaps mites including disjunct patterns of colony co-infection with Varroa destructor mites and the not dissimilar capacity of Tropilaelaps to vector viruses such as Deformed Wing Virus and Black Queen Cell Virus. It seems likely that Tropilaelaps will likely naturally displace Varroa wherever colonies maintain at least some brood year round because they breed more quickly, but that both mites will persist in a dynamic equilibrium in colder climes. Field rats and honey bees at the Gangstas Apiaries at Mataasna-Kahoy, Lipa in the Luzon district in the Philippines were the first discovered hosts of Tropilaelaps, specifically Tropilaelaps clareae, in 1961xxv, so rodents may be mite reservoirs elsewhere. However the actual source of mite re-emergence in regions where honey bees become broodless for extended periods has not been identified. Significantly the same researchers, Mercedes Delfinado and Edward Baker described a new family of honey bees mites, type species Euvarroa sinhai, on Apis florea from an apiary in New Delhi in 1971.xxvi
The future of Australian beekeeping in this new ‘mite age’ is anyone’s guess, especially in the purported absence of Deformed Wing Virus, but it seems certain that feral bee colony numbers will decline. Keeping bees successfully will come down to monitoring vigilance, to effective treatment and to good management practice. More critically it will need a more active program to breed Varroa tolerant bees and vigilance to prevent the incursion of Tropilaelaps mites.
We might also ask ourselves what behavioural traits have honey bees in general adopted to survive on their natural hosts. Giant, Asian and dwarf honey bees, as well as many African races of Apis mellifera, not only swarm regularly, they also abscond or routinely migrate. This behaviour results in extended periods of broodlessness, a strategy driven in part by nectar and pollen availability, by parasite and disease loading and by predators. The giant honey bees, for example, will return seasonally to the same nesting site but never employ old combs or nest in exactly the same position, a trait reducing their susceptibility to disease.
While cold temperate races of several species of honey bee rarely abscond, the practices of shook swarming and caging queens to force a broodless condition replicates their wild tropical race cousin behaviour. While cold climate honey bees typically enter a period of winter broodlessness, the population dynamics of both Varroa and Tropilaelaps mites are such that, unchecked, Varroa mites numbers blow out as colonies down regulate their numbers in autumn, while some Tropilaelaps mites somehow survive the coldest winters and come away quickly before colonies build in spring. Winter and spring colony losses due to mites can be very high.
Behavioural traits that have helped the natural hosts of mite parasites adapt to their presence is best illustrated amongst Asian honey bees (Apis cerana, Apis koschevnikovi, Apis nuluensis and Apis nigrocincta) that are naturally resistant to Varroa destructor, Varroa jacobsoni, Varroa rindereri and Varroa underwoodi. A combination of VSH (Varroa sensitive hygiene, in part grooming and decapping behaviour) and mite restriction to drone brood only development has accorded these honey bees a large measure of Varroa tolerance.
Overall we learn that European races of the Western Honey (Apis mellifera) have:
- developed considerable immunity – at least in Europe – to the tracheal mite, Acarapis woodi;
- ameliorated the impacts of both the varroa and tropi mites (Varroa destructor and Tropilaelaps mercedesae) through swarming, through absconding and through undergoing other natural periods of broodlessness;
- increased susceptibility to mite attack when colocated with or are within easy flying range of other hives especially when heavily infested; and
- been colocated with with other mite carrying species, notably tropi mite exchanges between western and giant honey bees.
However the history of mites jumping species is unlikely to be over. Both tracheal mite and tropi mites are now of recent serious concern to Apis cerana colonies in Japan while other mites have also also found new hosts (e.g. Euvarroa sinhai has migrated from Apis florea to its closely related cousin Apis andreniformis). According to Anderson and Baker, only two of the many genotypes of Varroa destructor (the Korean and the Japanese-Thai halotypes) have successfully migrated to Apis mellifera.xxvii
The ups and downs of taking a brood break
Various management interventions, chemical treatment apart, have been contrived by beekeepers to mimic natural behavioural responses to mites exhibited by the dozen of so honey bee species. Amongst these, avoiding co-location of hives – limiting the number of colonies in a single apiary and finding locations remote from other apiary operations – while admirable in principle, are often not achievable in practice. This said maintaining strict biosecurity measures may offer some reprieve to Tasmanian and western states in Australia and on Varroa-free islands.
Staintonxxviii is also one of many advocates of drone brood removal as an effective means of removal, though by no means full control, of mite populations. In practical terms this involves allowing the queen to lay out drone comb in a specially marked caged frame that is removed from the brood nest on a 21 day cycle. Such combs can either be full depth frames built on drone based foundation, or an empty with a starter strip that bees will draw out as drone comb. Alternatively a shallow or ideal frame with standard worker comb can be inserted into a full depth brood box (where bees will build drone comb below the bottom bar). In the latter case, drone brood can be scraped from the bottom bar periodically.
Brood cycle interventions
The notion of colony splitting, tweaked to induce an interruption to the brood cycles in both splits, as a partial solution to arresting mite production is intriguing. It is predicated on the fact that, in the absence of mite treatment, interruption to the brood cycle is the only measure that will halt mite breeding. How might we extend this practice to also control swarming and to requeen colonies, both measures that are conducive to building strong hives?
Many schemes employ the brood break principle. Amongst these shook swarming, artificial swarming (colony splitting) and queen caging can be implemented to some advantage. The downside of forcing a period of complete broodlessness is the loss of a brood cycle.
An intriguing strategy for Varroa control, one that has not been widely canvassed, is to employ a second queen to overcome the inherent setback that all brood interruption practices, including direct requeening, impose. In all instances brood break (or brood sacrifice), employed to deprive mites of sealed brood they need to reproduce, interrupts normal colony worker production. Traditionally uninterrupted brood production has been regarded as essential for development of powerful pollinator and honey gathering colonies. Employing a second queen to overcome this deficit is readily achievable in standard seasonal operations:
- in hive requeening where a second queen is established in a vertical split to control swarming and where both queens are retained before the colonies are reunited;
- in shook swarming where mite survival and their potential to reproduce is severely constrained; and
- in nuanced two-queen or doubled hive operations where very regular removal of drone comb can be practiced and where the power of a second laying queen is harnessed.
The first of these techniques has been adequately outlined by Stainton. The second shook swarming is a standard, though not routinely practiced, technique for controlling a range of diseases. It is a potential strategy for recovering colonies at risk of mite induced colony collapse.
So how else might we split hives, employing a second queen, to more effectively interrupt mite production? Practical schemes that take advantage of the laying power of a second queen include the aforementioned shook swarming, a scheme that exactly replicates natural swarming, and a modified Holzberlein scheme that we will come to. I have not trialled these interventions – Varroa is yet to arrive locally – but it seems they should work. Other nuanced variations in methods of splitting brood to effect mite control are outlined by Lilia de Guzman and coworkers in their published notes on the managment of Tropilaelaps mites.xxix
Shook swarming
The classic technique of shook swarming has been widely employed to reduce disease levels, for example to resolve chalk brood prevalence. It is especially effective as the colony with the old queen is forced into a cycle of broodlessness on disease free gear.
In shook swarming practice bees are shaken from diseased hives – though never those afflicted by American Foul Brood – into a fresh set of combs and fed if there is no honey flow. The potential of shook swarming to reduce mite population lies in the fact that the parent colony with all the brood must establish a new queen while the shook bees with the old queen must establish a new brood nest.
Here is the shook swarm plan (Figure 1). Set up a new bottom board with an empty super supplied with drawn comb – less ideally frames with foundation – on the parent hive stand and shake all bees in front of this hive. If the hive is very strong bees can be shaken into an additional empty super and similarly filled out with empty frames (Figure 1b, super C). As soon as this colony has settled and as quickly as possible remove the lid, add a queen excluder and place the bee-free broodnest on top to allow nurse bees to filter up through the excluder. An hour or so later again offset the parent hive, the unit with old combs, that will now contain sufficient bees to tend to the brood. Most field bees will be found in the shook swarm colony but will be broodless.
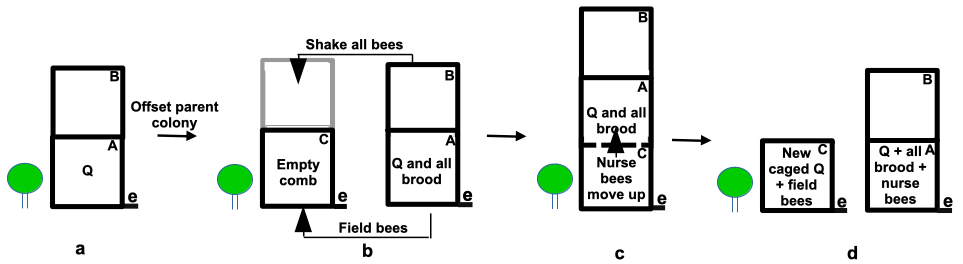
Figure 1 Shook swarming a strong colony: e = entrance; Q = queen:
(a) colony offset and all bees are shaken into an empty hive on the parent stand;
(b) add an excluder and shake all bees into the empty super (C);
(c) place brood boxes A and B on top of parent colony above an excluder to allow nurse bees to filter up to cover brood; and
(d) offset the colony now containing all the brood and many nurse bees. Add a caged queen or ensure a frame with eggs is present to allow the colony to raise its own queen.
The shook bees with the old queen will build a new brood nest from scratch and both colonies will be essentially broodless at different stages and can be treated accordingly to reduce mite loads. The offset parent hive will now be largely free of field bees and will become almost broodless by the time the colony raises a new queen, though a caged queen – if employed – will commence laying within a few days unless locked away. Treatment can then be instigated based on the level of mite infestation and the need or otherwise to isolate the new queen.
The two colonies, now each with a much reduced mite load, can be united on the parent stand leading into the honey flow. The old and inferior queen will be naturally superseded saving the need to locate and remove her. Conducted early enough in the season, the reunited colony will benefit from having had the presence of laying queens in each of the splits.
Holzberlein requeening and swarm control
To scope additional options that might work we might reflect on hive splitting practices that routinely induce a broodless condition in the queenless split and that have been effective in swarm control. In this we might consider a seemingly forgotten beekeeper Ralph Barnes from Oakland in California whose swarm control mantra was: ‘Divide or requeen’. The famed two-queen hive operator John Holzberleinxxx restated the Barnes’ scheme very eloquently:
…One is to swarm them, that is, some phase of dividing the old bees and queen from the young bees and brood. The other is to make the colony queenless and queen cell-less, causing it to raise a young queen from scratch.
Holzberlein concluded that neither measure were conducive to building bees for a honey flow. Instead he conceived a simple plan to co-opt the resources of an additional queen, a scheme that would both ‘divide and requeen bees’ simultaneously.
In the original plan, Holzberlein introduced a caged queen – rather than either a ripe queen cell or allowing the colony to raise its own queen – as the first step in establishing exceptionally powerful two-queen hives. His original plan was very explicit:
Set a strong colony back and in its place put a new bottom board and an empty brood chamber. Quickly sort the frames filling out this empty chamber with unsealed brood and some frames of honey. On top of this place an excluder and above that a further empty super returning the remaining brood combs and stores to this chamber after first shaking bees off these frames at the hive entrance. The queen will be in the bottom brood chamber and nurse bees will move up through the excluder to tend maturing and emerging brood. A day or two later the excluder is replaced with a double screen, an empty super is inserted and a new queen is introduced to the older brood and stores in the top super.
In his modified plan, however, his objective was far from that of establishing a giant colony with a large consolidated brood nest operating with two queens. His intent were to both control swarming and to later requeen by uniting the splits. He described his plan as ‘the best little swarm preventer that you ever tried’.
In order to facilitate mite control, we need simply to also cage the old queen and to treat both units around the twenty one day mark when all brood from the old queen will have emerged and ideally just before the new queen will have commenced laying.
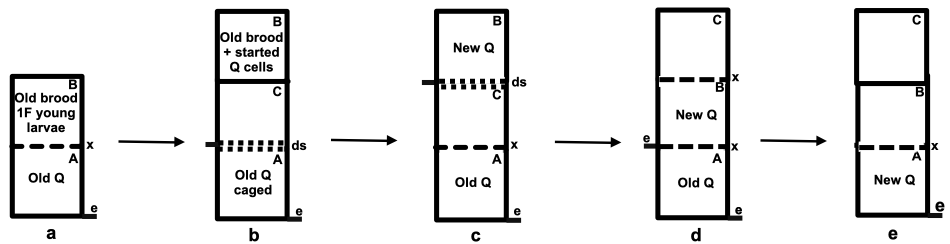
Figure 2 Modified Holzberlein split employing a second queen: e = entrance; Q = queen; ds = double screen; x = excluder:
(a) strong colony on the parent stand;
(b) the parent colony is split using double screen and by raising some stores, sealed brood and a single frame with eggs and young larvae to start a new queen;
(c) the new queen is allowed mate and to become well established;
(d) the two queens are allowed to lay until the beginning of the flow; and
(e) the colony reverts to the single condition for the flow.
In this smoke a mirrors scheme, mite reproduction is arrested for a brief period of near bloodlessness in each of the colonies (Figure 2, stages b and c). The subsequent later combined laying power of two queens (Figure 2d) more than makes up for losses that can be attributed to Varroa parasitism and periods of temporary broodlessness. Coming into the flow, the excluder is removed to allow the new queen to supersede the old queen.
You may think that employing two-queen systems to help overcome mite problems are far fetched. However Chilean researchers Villarroel, Rebolledo and coworkersxxxi demonstrated that doubled and tripled queened colonies helped address the problems of declining honey production attendant to the arrival of Africanised bees and Varroa. They achieved a 44% improvement in honey yield. In a parallel Chilean study, Valle, Guzmán-Novoa, Benítez, and Rubio employed the Moeller two-queen systemxxxii and doubled their honey yields. Despite the fact that running a second queen in any hive setup creates a giant brood-bank, a veritable haven for Varroa, this has not prevented more capable beekeepers running very successful operations using an additional queen.
The overall challenge will be to find no nonsense colony-building systems that are effective in controlling mites, but only ones easy to adopt in practice. Buying time, for example splitting strong hives in autumn rather than spring, and uniting them as early as late winter has been my past approach to get that early honey crop (with two queens). It may help resolve the problems of hive setback that Varroa control and other mites will inevitably incur.
Readings
iDelfinado, M.D. and Baker, E.W. (1974). Varroidae, a new family of mites on honey bees (Mesostigmata: Acarina). Journal of the Washington Academy of Sciences 64(1):4-10. https://www.jstor.org/stable/24535743
iide Guzman, L.I., Williams, G.R., Khongphinitbunjong, K. and Chantawannakul, P. (2017). Ecology, life history, and management of Tropilaelaps mites. Journal of Economic Entomology 110(2):319-332. doi:10.1093/jee/tow304 https://academic.oup.com/jee/article/110/2/319/3063341?login=false
iiiLocke, B. (2016). Natural Varroa mite-surviving Apis mellifera honeybee populations. Apidologie 47(3):467-482. doi.org/10.1007/s13592-015-0412-8 https://link.springer.com/article/10.1007/s13592-015-0412-8
ivRosenkranz, P. (1999). Honey bee (Apis mellifera L.) tolerance to Varroa jacobsoni Oud. in South America. Apidologie 30(2-3):159-172. doi.org/10.1051/apido:19990206 https://www.apidologie.org/articles/apido/abs/1999/02/Apidologie_0044-8435_1999_30_2-3_ART0006/Apidologie_0044-8435_1999_30_2-3_ART0006.html
vLe Conte, Y., de Vaublanc, G., Crauser, D., Jeanne, F., Rousselle, J.-C. and Bécard, J.-M. (2007). Honey bee colonies that have survived Varroa destructor. Apidologie 38(6):566-572. https://doi.org/10.1051/apido:2007040 https://link.springer.com/article/10.1051/apido:2007040#citeas
viKohl, P.L. and Rutschmann, B. (2018). The neglected bee trees: European beech forests as a home for feral honey bee colonies. Peerj 6:e4602 https://europepmc.org/article/PMC/PMC5890725
viiSeeley, T.D. (2007). Honey bees of the Arnot Forest: A population of feral colonies persisting with Varroa destructor in the northeastern United States. Apidologie 38(1):19-29. doi.org/10.1051/apido:2006055 https://link.springer.com/article/10.1051/apido:2006055#citeas
viiiOddie, M.A., Dahle, B. and Neumann, P. (2017). Norwegian honey bees surviving Varroa destructor mite infestations by means of natural selection. PeerJ 5:p.e3956. https://peerj.com/articles/3956/
ixRoberts J.M.K., Simbiken, N., Dale, C., Armstrong J. and Anderson D.L. (2020). Tolerance of honey bees to Varroa mite in the absence of deformed wing virus. Viruses 12:575. https://royalsocietypublishing.org/doi/full/10.1098/rspb.2021.1375 doi:10.3390/v12050575
xRinderer, T.E., de Guzman, L.I., Delatte, G.T., Stelzer, J.A., Lancaster, V.A., Kuznetsov, V.I.C.T.O.R., Beaman, L., Watts, R. and Harris, J.W. (2001). Resistance to the parasitic mite Varroa destructor in honey bees from far-eastern Russia. Apidologie 32(4):381-394. doi.org/10.1051/apido:2001138.
xiFries, I., Imdorf, A. and Rosenkranz, P. (2006). Survival of mite infested Varroa destructor honey bee Apis mellifera colonies in a Nordic climate. Apidologie 37(5):564-570. doi:10.1051/apido:2006031 https://www.apidologie.org/articles/apido/abs/2006/05/m6039/m6039.html
xiiLe Conte, Y., Meixner, M.D., Brandt, A., Carreck, N.L., Costa, C., Mondet, F. and Büchler, R. (2020). Geographical distribution and selection of European honey bees resistant to Varroa destructor. Insects 11(12):873. doi:10.3390/insects11120873
xiiiReyes-Quintana, M., Espinosa-Montano, L.G., Prieto-Merlos, D. Koleoglu, G., Petukhova, T., Correa-Benitez, A. and Guzman-Novoa, E. (2019). Impact of Varroa destructor and deformed wing virus on emergence, cellular immunity, wing integrity and survivorship of Africanized honey bees in Mexico. Journal of Invertebrate Pathology 164:43-48. https://sci-hub.hkvisa.net/10.1016/j.jip.2019.04.009
xivSammataro, D., Gerson, U. and Needham, G. (2000). Parasitic mites of honey bees: Life history, implications, and impact. Annual Review of Entomology 45(1):519-548. https://pubmed.ncbi.nlm.nih.gov/10761588/ https://www.researchgate.net/publication/12554109_Parasitic_Mites_of_Honey_Bees_Life_History_Implications_and_Impact
xvSakamoto, Y., Maeda, T., Yoshiyama, M. and Pettis, J.S. (2017). Differential susceptibility to the tracheal mite Acarapis woodi between Apis cerana and Apis mellifera. Apidologie 48(2):150-158. doi.10.1007/s13592-016-0460-8
xviMossadegh, M.S. (1990). Development of Euvarroa sinhai (Acarina: Mesostigmata), a parasitic mite of Apis florea, on A. mellifera worker brood. Experimental and Applied Acarology 9(1-2):73-78. doi:10.1007/bf01198984
xviiMartin, S.J. and Correia-Oliveira, M.E. (2016). The occurrence of ecto-parasitic Leptus sp. mites on Africanized honey bees. Journal of Apicultural Research 55(3):243-246. https://salford-repository.worktribe.com/preview/1493730/leptus20JAR20revised20submitted1.pdf doi:10.1080/00218839.2016.1228214
Flechtmann, C.H.W. (1980). Dois ácaros associados à abelha (Apis mellifera L.) no Perú [Two mites associated with the bee (Apis mellifera) in Peru]. Anais da Escola Superior de Agricultura Luiz de Queiroz 37(2):737–741. https://www.researchgate.net/publication/281320214_Dois_acaros_associados_a_abelha_melifera_Apis_mellifera_L_no_Peru
Southcott, R.V. (1989). A larval mite (Acarina: Erythraeidae) parasitizing the European honey bee in Guatemala. Acarologia 30(2):123-129. https://www.semanticscholar.org/paper/A-larval-mite-(Acarina%3A-Erythraeidae)-parasitizing-Southcott/3953661ef6c557cda25e3234d945334f189719fc?sort=relevance&page=2
Southcott, R.V. (1992). Revision of the larvae of Leptus latreille (Acarina: Erythraeidae) of Europe and North America, with descriptions of post-larval instars. Zoological Journal of the Linnean Society 105(1):1-153. doi.org/10.1111/j.1096-3642.1992.tb01228.x https://sci-hub.et-fine.com/10.1111/j.1096-3642.1992.tb01228.x
Quintero, M.M.T., Argote C,C. and Acevedo, H.A. (1987). Hallazgo de Pcaros del gtnero Leptus sp., parasitando a Apis mellifera. Veterinaria México 18(3):229-231.
Quintero, M.M.T., Argote C,C. and Acevedo, H.A. (1987). Mites of the genus Leptus sp parasitizing Apis mellifera short communication. Veterinaria México 18(3):229-231.
https://www.cabidigitallibrary.org/doi/full/10.5555/19910229872
Wilson, W.T., Wooley, T.A., Nunamaker, R.A. and Rubink, W.L. (1987). An erythraeid mite externally parasitic on honey bees (Apis mellifera). American Bee Journal 127(12):853-854.
Teixeira, É.W. (2008). Ocorrência de larvas de Leptus sp. Latreille 1796 (Acarina: Erythraeidae) em operárias de abelhas africanizadas A. mellifera Linnaeus 1758 (Hymenoptera: Apidae), no Brasil. Boletim de Indústria Animal 65(3):249-251. http://bia.iz.sp.gov.br/index.php/bia/article/view/1130 http://bia.iz.sp.gov.br/index.php/bia/article/view/1130/1124
xviiide Guzman, L.I., Williams, G.R., Khongphinitbunjong, K. and Chantawannakul, P. (2017) loc. cit.
Anderson, D.L. and Morgan, M.J. (2007) loc. cit.
xixFranco, S. (2018). OIE Terrestrial Manual, Chapter 3.2.6. Infestation of honey bees with Tropilaelaps spp. pp.765-776. OIE World Organisation for Animal Health. https://www.oie.int/fileadmin/Home/fr/Health_standards/tahm/3.02.06_TROPILAELAPS.pdf
xxWade, A (2022). Highways and byways of beekeeping: Phoretic honey bee mites, p.310. Northern Bee Books, Scout Bottom Farm, Mytholmroyd, West Yorkshire.
xxiAnderson, D.L. and Morgan, M.J. (2007). Genetic and morphological variation of bee-parasitic Tropilaelaps mites (Acari: Laelapidae): New and re-defined species. Experimental and Applied Acarology 43(1):1-24. https://openresearch-repository.anu.edu.au/bitstream/1885/51418/2/01_Anderson_Genetic_and_morphological_2007.pdf https://pubmed.ncbi.nlm.nih.gov/17828576/doi:10.1007/s10493-007-9103-0
xxiiChantawannakul, P., Ramsey, S., vanEngelsdorp, D., Khongphinitbunjong, K. and Phokasem, P. (2018). Tropilaelaps mite: An emerging threat to European honey bee. Current Opinion in Insect Science 26:69-75. doi:10.1016/j.cois.2018.01.012 https://www.sciencedirect.com/science/article/abs/pii/S2214574517300810
xxiiiRobert, S. (March 2023). Newsround: Yet another pest. The Beekeepers Quarterly 151:8.
xxivChantawannakul, P., Ramsey, S., vanEngelsdorp, D., Khongphinitbunjong, K. and Phokasem, P. (2018) loc cit.
xxvDelfinado, M.D. and Baker E.W. (1961). Tropilaelaps, a new genus of mite from the Philippines (Laelapidae [s. lat.], Acarina). Fieldiana Zoology 44(7):53-56. https://www.biodiversitylibrary.org/partpdf/36189
xxviDelfinado, M.D. and Baker, E.W. (1974). Varroidae, a new family of mites on honey bees (Mesostigmata: Acarina). Journal of the Washington Academy of Sciences 64(1):.4-10. https://www.jstor.org/stable/24535743
xxviiAnderson, D.L. (2002). Varroa-bee relationships – what they tell us about controlling Varroa mites on the European honey bee. Apiacta 3:9-11. http://www.fiitea.org/cgi-bin/index.cgi?sid=&zone=cms&action=search&categ_id=53&search_ordine=descriere http://www.fiitea.org/foundation/files/2002/D.L.%20ANDERSON.pdf
xxviiiStainton, K. [United Kingdom Perbright Institute] (2022) . Varroa management: A practical guide on how to manage Varroa mites in honey bee colonies. Northern Bee Books.Scout Bottom Farm, Mytholmroyd, West Yorkshire.
xxixde Guzman, L.I., Williams, G.R., Khongphinitbunjong, K. and Chantawannakul, P. (2017) loc. cit.
xxxHolzberlein Jr., J.W. (May 1952). Swarm prevention—not swarm control. American Bee Journal 92(5):195-196. https://archive.org/details/sim_american-bee-journal_1952-05_92_5/page/195/mode/1up
xxxiVillarroel, D.T., Rebolledo, R.R. and Aguilera, A.P. (1998). Comparative study of honey production with one and two queens per hive in the area of Nueva Imperial, IX Region, Chile. [Estudio comparativo de producción de miel con una y dos reinas por colmena en la zona de Nueva Imperial, IX Región, Chile.] Agro Sur 26(2):121-126. http://revistas.uach.cl/html/agrosur/v26n2/body/art12.htm
Rebolledo, V.R.R., Guiñez, C.G., Araneda, D.X. and Aguilera P.A. (2008). Comparative study of honey bee production with one and three queens by beehive in Neuva Imperial, IX Region, Chile. [Estudio comparativo de producción de miel con una y tres reinas por colmena en la zona de Nueva Imperial, Chile.] Idesia 26(2):19-25. https://scielo.conicyt.cl/scielo.php?pid=S0718-34292008000200004&script=sci_arttext and https://www.researchgate.net/publication/297710235_Comparative_study_of_honey_bee_production_with_one_and_three_queens_by_beehive_in_nueva_imperial_IX_region_Chile
Rebolledo, R.R., Riquelme, M.C., Haiquil, S., Sepúlveda, G. and Aguilera, A.P. (2011). Comparative study of honey and pollen production in a double queen system versus one queen per hive in La Araucanía Region, Chile. [Estudio comparativo de la producción de polen y miel en un sistema de doble reina versus una por colmena en La Araucanía, Chile.] Idesia 29:139-144. http://www.Idesia.cl/Vols/2011/29-2/art18.pdf and https://scielo.conicyt.cl/ scielo.php?script=sci_arttext&pid=S0718-34292011000200018&lng=en&nrm=iso&tlng=en
Cengiz, E.H., Genç, F. and Cengiz, M.M. (2019). The effect of the two-queen colony management practice on colony performance and Varroa (Varroa destructor Anderson&Trueman) infestation levels in honey bee (Apis mellifera L.) colonies. Uludag Bee Journal 19(1):1-11. https://dergipark.org.tr/en/download/issue-full-file/45334
xxxiiValle, A.G.G., Guzmán-Novoa, E., Benítez, A.C. and Rubio, J.A.Z. (2004). The effect of using two bee (Apis mellifera L.) queens on colony population, honey production, and profitability in the Mexican High Plateau. Téc Pecu Méx 42(3):361-377. http://cienciaspecuarias.inifap.gob.mx/index.php/Pecuarias/article/viewFile/1404/1399
