Alan Wade
Phoresis or phoresy is a non-permanent, commensalistic interaction in which one organism (a phoront or phoretic) attaches itself to another (the host) solely for the purpose of travel… Phoresis is rooted in the Greek words phoras (bearing) and phor (thief).
There are of the order of a million species of mites, the majority of which are un-described. While the vast majority of these mites are free living (macrophages and detrivores) many others are less benign. A number are serious pests of vertebrates, plants and insects including, of course, bees.
Phoretic mites are hitchhikers. They attach themselves to their host, in our very special case to honey bees. This aids in both their dispersal and survival. Relatively few bee-associated mites are parasitic, many being commensal (mutually beneficial) beehive occupants.
Honey bee mites, like all arachnids, are wingless so must crawl into, or hitch a ride to, beehives to consume comb, bee debris and fungal material (Table 1). As noted by Hepburn and Radloffi:
Parasitic mites represent only a minor fraction of the diversity of Acari associations with honeybees. Most Acari found in the nests of honeybees usually have a saprophagous lifestyle and feed on fungus-infected debris in the hives, dead bees and sometimes pollen (kleptophages).
| Mite order | Mite species | Bee-mite association |
| Astigmata | Forcellinia faini | Scavenger of bee and insect debris and fungi |
| Prostigmata | Pseudacarapis indoapis | Pollen feeder in Apis cerana colonies |
| Mesostigmata | Melichares dentriticus | Predator of scavenger mites |
| Melittiphis alvearius | Globally distributed pollen feeding mite in Apis mellifera colonies | |
| Afrocypholaelaps africana and other species | Flower mites phoretic on honey bees | |
| Neocypholaelaps spp | Flower mites phoretic on honey bees |
Table 1 Commensal mites associated with honey bees. There are many others, see for example Refaei and coworkersii. Mites of Australian native bees are also knowniii.
Mites as arachnids
Let’s start by examining the larger group to which the mites belong. Mites are all arachnids and all are jointed, eight-legged invertebrates. Arachnids are easily distinguished from insects: insects have six legs, wings and antennae. Next time you pick up a spider or a scorpions – or remove a tick – check it out to make sure it does not have wings or a fewer or greater numbers of legs.
Fleas have their own way and so do mites:
The vermin only tease and pinch
Their foes superior by an inch.
So, naturalists observe, a flea
Has smaller fleas that on him prey;
And these have smaller still to bite ’em.
And so proceed ad infinitum.
The poems of Jonathon Swift, Volume 1, 1733
On poetry – A rhapsody. p.274.
The arachnids – aráchni is spider in Greek – have a close affinity with the Chelicerae (Figure 1) having descended from their marine ancestors. As you can see they are related to present day horseshoe crabs and sea spiders.
The Chelicerae are in turn a subphylum of the vast invertebrate group Arthropoda – jointed leg invertebrates – that encompasses the insects, crustacea, centipedes and millipedes.
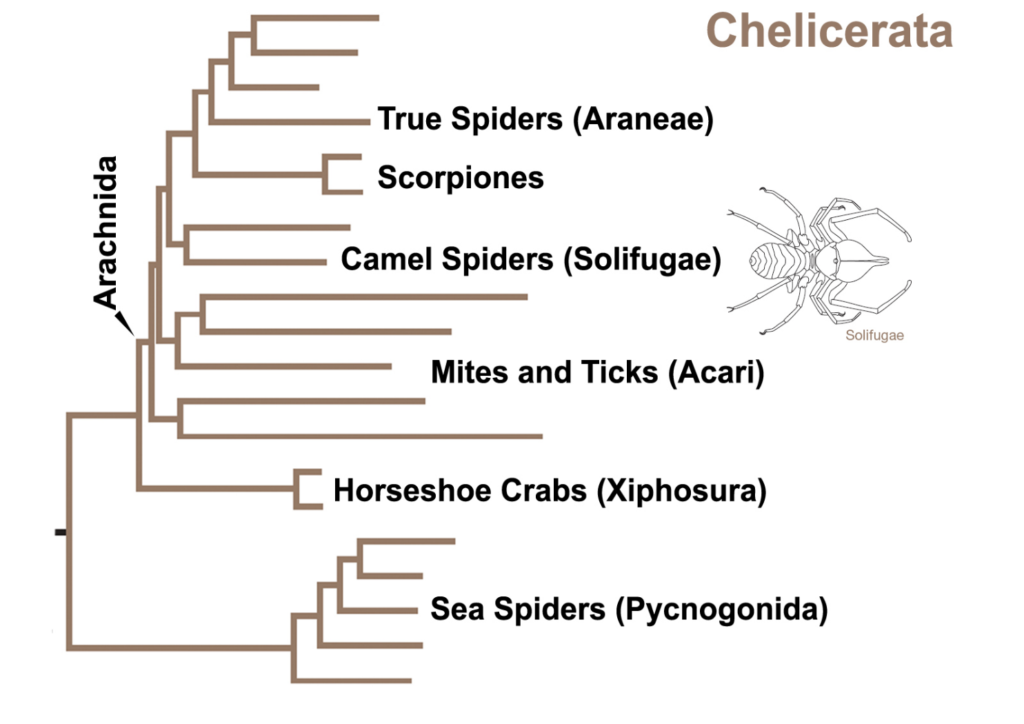
Figure 1 Relationship of arachnids such as mites to jawed or fanged arthropods (Chelicidae)
Drilling further down we discover that the mite group belong to a major subclass of arachnids, the Acari (Figure 2). The Acari comprise two distinctive lineages, the superorders Acariformes and the Parasitiformes. Together, the Acari are clearly delineated from spiders, scorpions, daddy longlegs and solifuges shown to the right of the chart.
There are a multitude of mites, perhaps a million of them, found in habitats as diverse as the deep oceans, deserts, poles and the upper atmosphere. So when you hear beekeepers talk about ‘the mite’ they are probably understating their diversity.
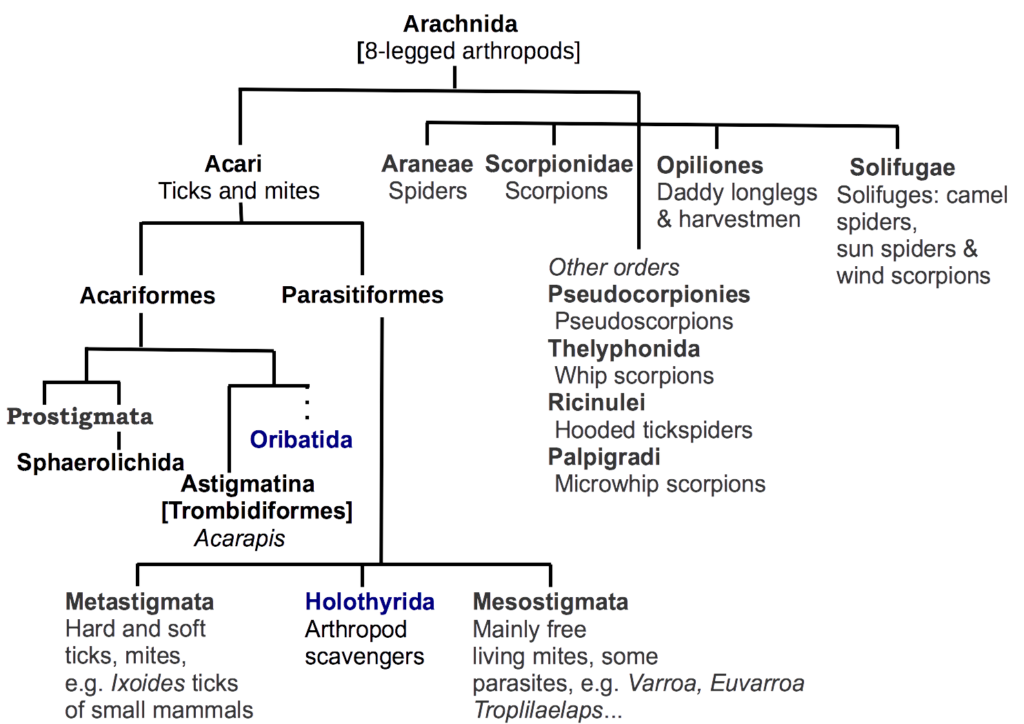
Figure 2 Simplified arrangement of the major orders of 8-legged arachnids (Class: Arachnida)
In examining this assemblage I recall a visit I made to the Jenolan Caves back in the late 1960s. An enthusiastic guide, eager for us to recall the difference between stalactites and stalagmites, provided the ultimate mnemonic: ‘When the mites come up, the tights come down’.
Phoretic mites
Mites plague vertebrates and arthropods alike and honey, bumble, stingless and solitary bees are no exception. Many arrive as uninvited hitchhikers, the so-called phoretic mites.
Unlike the myriad honey bee pests that can literally fly in the front door – hive beetles, Braula fly, wax moths, and vespid wasps and hornets – the honey bee phoretic mites rely on normal behaviours of bees, robbing, foraging and swarming to gain hive entry. There is little beyond culling infested colonies and drastic treatment you can do to keep these mites at bay. A good rule of thumb is to locate your bees well away from other, often poorly managed, apiaries that your healthy bees will be inclined to raid.
Predatory honey bee mites, all phoretic, fall into the Astigmatina and Mesostigmata arachnid ordersiv. Their natural hosts are listed in Table 2. They have evolved to feast on haemolymph (bee blood) but, with the exception of the tracheal mite, have developed fairly effective defence strategies such as grooming and biting behaviour. A notable example of successful coexistence is that of Varroa mite has on its well-adapted original host Apis cerana.
| Mite order | Mite family | Parasite species | Original host species |
| Astigmatina | Tarsonemidae | Acarapis woodi [Tracheal mite] | Apis mellifera |
| Acarapis externus Acarapis dorsalis [External mites]v | Not known but found on Apis dorsata, Apis cerana and Apis mellifera in Asia | ||
| Mesostigmata | Varroidaevi | Varroa destructor [Varroa mite] | Apis cerana |
| Varroa jacobsoni Varroa rindereri Varroa underwoodi | Likely Apis cerana Apis koschevnikovi Apis cerana | ||
| Euvarroa wongsirii Euvarroa sinhai | Apis andreniformis Apis florea | ||
| Laelapidaevii | Tropilaelaps clareae Tropilaelaps mercedesae Tropilaelaps koenigerum Tropilaelaps thaii | Apis breviligula Apis dorsata Apis laboriosa |
Table 2 Phylogenic relationships of predatory mites with honey bees
Broadly speaking:
- Acarapis mites appear to have evolved with the Western (one mite species) and Asian Honey bees (two mite species);
- Varroa mites have evolved within the Asian honey bee group that includes Apis cerana;
- Euvarroa mites have evolved with the dwarf honey bees; and
- Tropilaelaps mites have evolved with the giant honey bees.
The mighty mites
Mite-bee host relationships are extremely complex especially in terms of their cross over to other honey bee species. For example Apis koschevnikovi, the natural host of Varroa rindereri, has spread to Apis cerana and to Apis nuluensis but not as yet to Apis nigrocinta or to Apis mellifera. Excellent overviews of the interactions between these mites and the honey bees in the Asian region are provided by Chantawannakul et alviii and by Warrit and Lekprayoonix.
Varroa mite jumping species to unadapted Apis mellifera is an exemplar of animal-parasite imbalance. Ebola, Hendra and Corona viruses moving from bats to vertebrate hosts, not just to human beings, may ring a bell.
That brings us to the serious honey bee pest status of phoretic mites (Table 3). The cross-species movement of these parasites is complex and each group of mites is considered in turn.
Acarapis (Acarine) mites
The Acarapis honey bee parasite group belongs to the Acariformes arm of the Acari. The origin of the tracheal mite Acarapis woodi, the causitive agent of Acarine (Isle of Wight) disease, is unknown but it appeared on European honey bees, where it was fist realised to be problematic, on the Isle of Wight in 1904. It was not formally identified as a tracheal mite until 1922. Sammataro, Gerson, and Needhamx argue that its subsequent finding that is more widespread dispels the notion that it suddenly evolved in Europe. More recently Acarine disease has had a devastating impact on the Asian Honey Bee reportedly causing destruction of Apis cerana japonica colonies in Japan.
| Mite name | Mite species | Mite pest status |
| Acarine Disease, Tracheal Mite, Isle of White Disease | Acarapis woodi | Serious pest of Apis mellfera and, in southern Asia, Apis cerana |
| External Acarapis Mite | Acarapis dorsalis Acarapis externus | Minor pests of Apis dorsata, Apis cerana and Apis mellifera |
| Varroa Mite | Varroa destructor | Serious global pest of Apis mellifera and Apis cerana |
| Varroa Mite | Varroa jacobsoni Varroa rindereri Varroa underwoodi | Pests of native Apis cerana, Apis koschevnikovi and Apis cerana respectively |
| Euvarroa Mite | Euvarroa wongsirii Euvarroa sinhai | Pests of native Apis florea and Apis andreniformis |
| Tropilaelaps Mite, Asian Mite | Tropilaelaps clareae Tropilaelaps koenigerum | Serious pests of Apis mellifera and Apis cerana but spread is currently limited to southern Asia |
| Tropilaelaps Mite, Asian Mite | Tropilaelaps mercedesae and Tropilaelaps thaii | Appear to be mainly pests of native Apis dorsata brevigula and Apis dorsata laboriosa but have spread mainly to other Apis dorsata subspecies |
Table 3 Parasitic mites of honey bees.
The evolutionary origins of the related ectoparasites Ascarapis dorsalis and Ascarapis externus found in southern Asia are similarly obscure. They, too, appear to have a wide natural distribution being found on Apis dorsata, Apis cerana and introduced Apis mellifera. Their observed impact on honey bees has been minimal.
Varroa mites
Varroa mites have evolved with the Asian Honey Bee, Apis cerana, and its close relatives, Apis nululensis, Apis nigrocincta and Apis koschevnikovi.
Varroa destructor is native to Apis cerana on the Asian mainland. It has jumped to Apis mellifera twice reflected in the circulating Korean and Japanese-Thai halotypes. There are a number of other Varroa destructor halotypes so only a critical few impact on Apis mellifera. This said, the global impact of Varroa destructor (Varroa mite) has been devastating amplified in large measure by bee viruses. Of these Deformed Wing Virus has been most widely cited as the prime cause of colony collapse, though this has been disputed by the University of Sydney’s Madeleine Beekman.
This Varroa mite appears to have been much less destructive to some African races of the Western Honey Bee. Interestingly Tom Seeleyxi has shown that while the mite initially decimated wild honey bee colonies in New York State, that is after its arrival, colony numbers quickly returned to pre-Varroa levels. Seeley attributes their resurgence to wide separation of wild bee nests – where robbing is less problematic, frequent swarming and to the relatively small size of wild colonies (where the potential to build up large mite populations is minimal).
Efforts to breed Varroa resistant strains of honey bees has met with very limited success while attempts to relocate pockets of varroa-resistant bees has so far been similarly unsuccessful. One awaits the outcome of ABC news reports of David Briggsxii working to artificially inseminate Varroa-resistant material into Australian queen bee stock to make beekeeping here Varroa ready.
Varroa jacobsoni is native to Sumatra, Java, Kalimantan and surrounding islands. It is now widespread across central and southern Thailand, Malaysia, Indonesia, Palawan Island and Papua New Guinea. Varroa jacobsoni natural hosts are Apis cerana and the Philippine Honey Bee Apis nigrocincta. It has been found on Apis mellifera but its impact and status on the Western Honey Bee has been uncertain and the subject of some debate.
Varroa underwoodi is found on Apis cerana, Apis nigrocincta and Apis nuluensis and is known from bees found in the Sulawesi and Kalimantan.
Varroa redereri has only been found on Apis koschevnikovi and is found in Malaysia and western Indonesia including Kalimantan.
Euvarroa mites
These mites evolved with and are largely restricted to the dwarf honey bees. The natural host of Euvarroa wongsirii is the southeastern Asian dwarf honey bee Apis andreniformis but it is now found on the more westerly and more widely distributed Apis florea.
Euvarroa sinhai is the natural host is Apis florea. It has not moved across to Apis andreniformis with which it is sympatric (meaning their ranges overlap) in Myanmar, Thailand and West Malaysia. It has been reported in Apis mellifera colonies though not accorded pest status.
Tropilaelaps mites
There are four Tropilaelaps species. All are naturally hosted by the giant honey bee Apis dorsata of which there are also four recognised geographical variants. Apis dorsata laboriosa is one of two very distinctive forms of giant honey bee. How and when the different Tropilaelaps species and the regional variants of Apis dorsata evolved or diverged is unknown, but some recognition of the geographical variability of their distribution may be key to understanding their impact on both the cultivated Asian and Western Honey Bees.
Apis dorsata laboriosa or Apis laboriosa, the Himalayan Cliff Honey Bee, is the largest of all living honey bees. Its isolation and its adaptation to high altitude lends support to its being accorded separate species status. This bee never harvests abandoned nest wax and hive materials and avoids the same rock attachment on return to the same cliff overhang after overwintering in forests at lower altitude.
The other really distinctive subspecies is Apis mellifera breviligula – found in most parts of the Phillipines. It is sometimes also accorded separate species status.
The grooming behaviour of the giant honey bee(s), as well as their propensity to migrate – resulting in breaks in the breeding cycle – greatly reduces parasite impact.
The most coherent account of Tropilaelaps mites, their associations with honey bees and their impacts is provided by de Guzman et alxiii. Quoting from elsewhere they note ominously:
Varroa and Tropilaelaps mites have coexisted in Apis mellifera colonies in Asia for more than fifty years and that Tropilaelaps mites are considered to be the more dominating and reproductively successful parasites of Apis mellifera than Varroa mites.
Lilia de Guzman and coworkers go on to note that Tropilaelaps mercedesae and Tropilaelaps thaii are closely related being genetically separate from similarly closely related Tropilaelaps clareae and Tropilaelaps koenigerum.
The widely distributed Tropilaelaps mercedesae is found across mainland Asia [Afghanistan, China, India, Indonesia (except the Sulawesi), Kenya, Laos, Malaysia, Myanmar, Nepal, Pakistan, Papua New Guinea, South Korea, Sri Lanka, Thailand and Vietnam] and, more ominously for Australia, in the Palawan Islands in the Phillipines in the South China Sea and in islands neighbouring Australia [Indonesia excluding the Sulawesi in Indonesia and in Papua New Guinea (PNG)]. In PNG it is found on Apis mellifera despite the region being free of giant honey bees.
According to former CSIRO scientist Denis Anderson and Matthew Morgan Tropilaelaps clarae appears to be much more restricted being found only in the Sulawesi in Indonesia and the Phillipines outside the Palawan Islandsxiv. Tropilaelaps mercedesae, with which Tropilaelaps clarae has been confused, is considered to be the mite most threatening to the global beekeeping industry.
Tropilaelaps koenigerum is a parasite of Apis dorsata breviligula (Asia including Indonesia except the Sulawesi). It is now also a parasite of Apis dorsata laboriosa and Apis mellifera.
Tropilaelaps thaii is a parasite of Apis dorsata laboriosa and originated in Vietnam. It is now widely distributed in South and Southeast Asia and is found mainly in forested areas of Nepal, Malaysia and Singapore.
The jury is out
If you are a little confused as to which mite infects which honey bee and the future of honey bees, you are in good company. I am of the view that some of the lesser known honey bees are, themselves, threatened, mainly from loss of habitat and human predation. I have trouble concurring with the widespread view that Apis mellifera (the Western Honey Bee) and perhaps also Apis cerana (the Asian Honey Bee) are under imminent threat, despite widespread managed colony losses.
The apiculture industry is certainly seriously impacted, but the continued survival and recovery of wild populations of these keystone species suggests that they are adapting to the parasites. The greater risk to bees would appear to revolve around well intended but perhaps mistaken use and reliance on miticides for their control.
Randy Oliver from Scientific Beekeeping is of the view that chemical control of mites may be exacerbating their potential impact. In much the same way antibiotic control of Paenobacillus larvae, the causitive agent of American foulbrood, only masks its survival and spread. So use of miticides to control Varroa mite may be breeding mite resistance and giving free reign to development of more virulent mite-associated viruses.
A few parting thoughts. Most recent interceptions of exotic honey bees and their mites to Australian shores has been associated with commercial shipping from fairly distant ports. However Papua New Guinea and Indonesia to our near north bristle with exotic honey bees and pest mite species, so the risk of their island hopping through the Torres Strait and by boat from Nusa Tengarra, the Indonesian southeastern islands, is existential.
Readings
iWarrit, N. and Lekprayoon, C. (2011). Asian honeybee mitesin Honeybees of Asia. (Hepburn, H.R. and Radloff, S.E. [eds]), Chapter 16, p.359. Springer-Verlag Berlin Heidelberg. https://www.bijenhouders.nl/files/Bijengezondheid/Cornelissen/Warrit_Lekprayoon_2011_Asian%20honey%20bee%20mites%20(1).pdf
iiRefaei, G.S., Zeid, W.R.A. and Roshdy, O.M. (2018). Incidence of parasitic and non-parasitic mites of honeybee, Apis mellifera (Linnaeus). Journal of Plant Protection and Pathology 9(12):873-875. DOI: 10.21608/jppp.2018.44098
iiiWalter, D.E., Beard, J.J., Walker, K.L. and Sparks, K. (2002). Of mites and bees: A review of mite–bee associations in Australia and a revision of Raymentia Womersley (Acari: Mesostigmata: Laelapidae), with the description of two new species of mites from Lasioglossum (Parasphecodes) spp. (Hymenoptera: Halictidae). Australian Journal of Entomology 41(2):128-148. https://doi.org/10.1046/j.1440-6055.2002.00280.x
ivWalter, D.E., Krantz, G. and Lindquist, E. (1996). Acari: The mites. http://tolweb.org/acari
Linquist, E.E. (1984). Current theories on the evolution of major groups of Acari and on their relationships with other groups of Arachnida, with consequent implications for their classification. Proceedings of the 6th International Congress of Acarology, Edinburgh, Scotland, Sept. 1982. Vol. 1. Chichester, England, Ellis Horwood Ltd, pp. 28–62. http://museum.wa.gov.au/catalogues-beta/bibliography/current-theories-evolution-major-groups-acari-and-their-relationships-other-groups
vDelfinado-Baker, M. and Baker, E.W. (1982).Notes on honey bee mites of the genus Acarapis hirst (Acari: Tarsonemidae). International Journal of Acarology 8(4): 211-226. http://www.tandfonline.com/doi/abs/10.1080/01647958208683299?journalCode=taca20
viDelfinado, M.D. and Baker, E.W. (1974). Varroidae, a new family of mites on honey bees (Mesostigmata: Acarina). Journal of the Washington Academy of Sciences 64(1):4-10. https://www.jstor.org/stable/24535743
viide Guzman, L.I., Williams, G.R., Khongphinitbunjong, K. and Chantawannakul, P. (2017). Ecology, life history, and management of Tropilaelaps mites. Journal of Economic Entomology 110(2):319–332. doi: 10.1093/jee/tow304
viiiChantawannakul, P., de Guzman, L.I., Li, J. and Williams, G.R. (2016). Parasites, pathogens, and pests of honeybees in Asia. Apidologie 47(3):301-324. https://hal.archives-ouvertes.fr/hal-01532338/document
ixWarrit and Lekprayoon (2011) loc. cit.
xSammataro, D., Gerson, U. and Needham, G. (2000). Parasitic mites of honey bees: Life history, implications, and impact. Annual Review of Entomology 45:519–548. https://www.ars.usda.gov/ARSUserFiles/31186/ann.rev.samm.pdf
xiSeeley, T. (2019). The lives of bees: The untold story of honey bees in the wild. Princeton University Press.
xiiLee, T. (Fri 16 Jul 2021). Dutch honey bees resistant to varroa mite imported to Australia to help guard against the pest. ABC Landline. https://www.abc.net.au/news/2021-07-16/bee-imports-to-protect-against-varroa-mite/100289356
xiiide Guzman, L.I. Geoffrey R. Williams, G.R., Khongphinitbunjong, K. and Panuwan Chantawannakul, P. (2017). Ecology, life history, and management of Tropilaelaps mites. Journal of Economic Entomology 110(2):319–332. https://doi.org/10.1093/jee/tow304
xivAnderson D.L., Morgan M.J. (2007). Genetic and morphological variation of bee parasitic Tropilaelaps mites (Acari: Laelapidae): New and re-defined species. Experimental and Applied Acarology 43:1–24. https://link.springer.com/article/10.1007/s10493-007-9103-0
