Alan Wade
As in other animals, there are two types of glandular chemicals that regulate honey bee behaviour: primer pheromones and releaser pheromonesi.
Primer pheromones act at a physiological level, triggering complex and long-term responses in the receiver and generating both developmental and behavioural changes. Releaser pheromones have a weaker effect, generating a simple and transitory response that influence the receiver only at the behavioural level. As well there are a range of natural scents such as those produced by flowers, that bees quickly learn to recognise.
Let us start with the influence of these chemical agents inside the hive. There we can examine the range of bee glands that release these pheromones, noting that they control activities as disparate as comb construction and brood rearing.
Queen mandibular pheromone
When the principle queen mandibular pheromone 9-ODA was first isolated and synthesised by Colin Butler and coworkersii at the Rothamsted Agricultural Experiment Station in 1962, our understanding of chemical communication in honey bee colonies was revolutionised. However the finding was woefully lacking in detail: much more is now known and other mandibular pheromones were soon discoverediii.
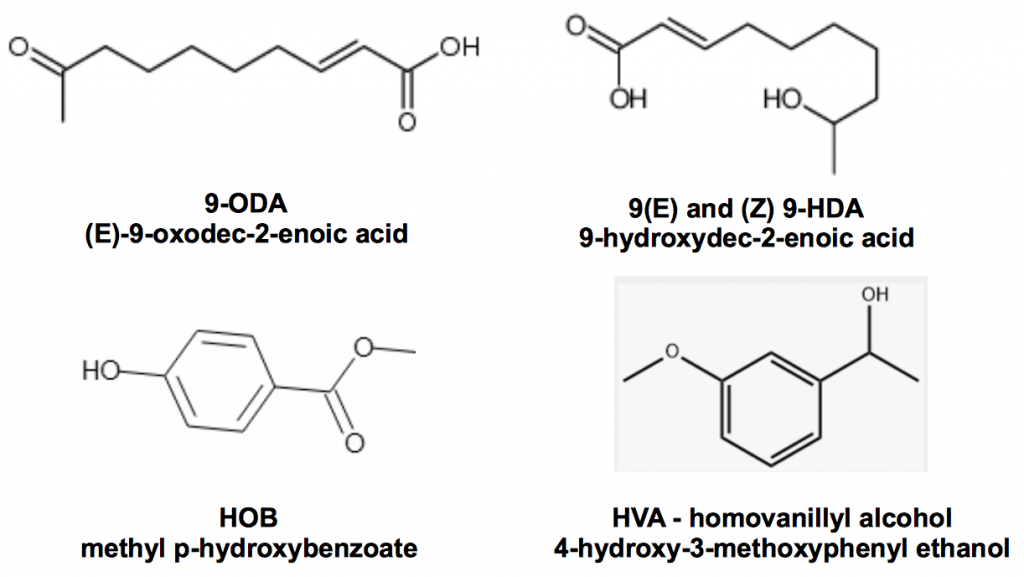
Figure 1 Queen mandibular pheromone complex, 9-ODA, the trans and cis enantiomers of 9-HDA, HOB and HVA
Slessor, Kaminski, King, Borden, and Winston outline the very specific chemical basis for retinue response of worker bees to the presence of a queeniv:
The substance produced by the mandibular gland complex of queen honey bees is considered to be responsible for the retinue formation in honey bees, Apis mellifera L. The retinue response includes the licking and atennation behaviour which signals the presence of a dominant reproductive queen and thereby establishes and stabilizes the social fabric of the colony…
They go on to describe how that each of the constituents found in mandibular extracts elicits very weak retinue behaviour but that, together with 9-ODA, they perform the full function even though some of these chemicals are present at vanishingly low concentrations. There are a plethora of other controls effected by queen mandibular pheromone, for example the regulation of construction of drone comb and prevention of raising of new queens. Sufficient to say is that a young and well-mated queen runs a very tight ship.
Interestingly Paul Hurd at Queen Mary University of London has outlined the importance of the epigenetic effect of the pheromone HDA (9-hydroxydecenoic acid) in royal jelly in influencing caste development (queen versus worker) in honey bee larvae. By day four after eggs hatch, the pathway to queen development is set while that of the worker is far from complete.
Interestingly Paul Hurd at Queen Mary University of London has outlined the importance of the epigenetic effect of the pheromone HDA (9-hydroxydecenoic acid) in royal jelly in influencing caste development (queen versus worker) in honey bee larvae. By day four after eggs hatch, the pathway to queen development is set while that of the worker is far from complete.
Here is a short video I recorded of this behaviour: bees groom the queen licking her and transferring 9-ODA and the adjuvant pheromones to nest mates, by and large by trophallaxis, that is mouth-to-mouth — proboscis-to-proboscis — exchange of food and regurgitated salivary material:
Tracking laying honey bee queen in Canberra Region Beekeepers observation hive 24 May 2019
Bortolotti and Costav provide a wider overview of the influence of this mixture of chemicals:
The honey bee queen represents the main regulating factor of the colony functions. This regulation is largely achieved by means of pheromones, which are produced by different glands and emitted as a complex chemical blend, known as the queen signal.
The queen signal acts principally as a primer pheromone, inducing several physiological and behavioral modifications in the worker bees of the colony that result in maintenance of colony homeostasis through establishment of social hierarchy and preservation of the queen’s reproductive supremacy. More specifically, the effects of the queen signal are maintenance of worker cohesion, suppression of queen rearing, inhibition of worker reproduction, and stimulation of worker activities: cleaning, building, guarding, foraging, and brood feeding.
Maissonaise and coworkersvi go further suggesting that queen mandibular pheromone alone:
…does not trigger the full behavioral and physiological response observed in the presence of the queen, suggesting the presence of additional compounds.
Using other queen body part extracts they found that additional chemicals were able to exert additional influence on the control of worker bee ovary development, comb construction and worker bee queen attendance (retinue behaviour). Keeling and coworkersvii identified yet more chemicals produced by glands other than queen mandibles (Figure 2) that enhance queen bee retinue formation. While each of these chemicals alone were elicited no response together they could together still attract workers even in the absence of queen mandibular pheromoneviii. We can conclude that chemical signalling is a complex process not always limited by the presence or absence of a specific pheromone.
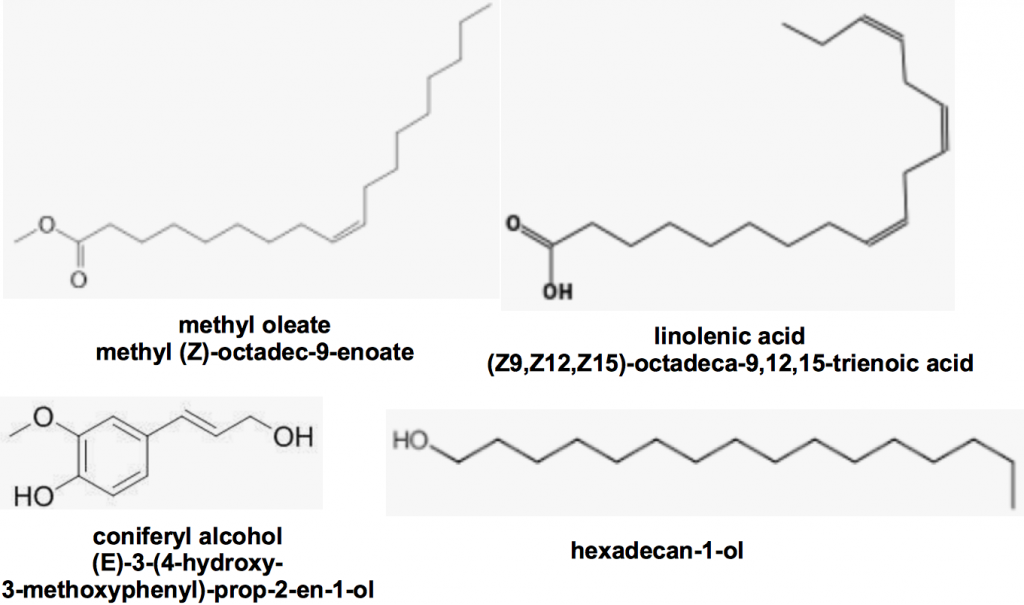
Figure 2 Queen retinue pheromones produced by glands other than queen mandibles
Devolved decision making by worker and drone bees
Despite the common beekeeper perception of the supremacy of the queen in the honey bee colony, the colony itself – and largely the worker bees – exercise an enormous influence over other facets colony functioning. One may think of the colony as a large organism with facility for workers to regulate and direct such complex tasks as foraging, comb building in response to nectar inflow, brood raising and queen replacement as well as swarm initiation largely independent of any queen signalling.
Let’s restrict the discussion to specific instances where chemical signalling can be traced to bees other than the queen beginning with the brood itself and then exploring the realm of both workers and drones in controlling behaviours both at the colony and individual bee level within and outside the hive.
Brood pheromone
Brood pheromones comprising straight chain methyl and ethyl esters of saturated and unsaturated fatty acids are well knownix to inhibit worker bee ovary development and to enhance worker foraging behaviour. Queen mandibular pheromones play a similar role in controlling the laying worker ovary development, with the unsaturated terpene (E)-β-ocimenex being added to the list of active constituents.
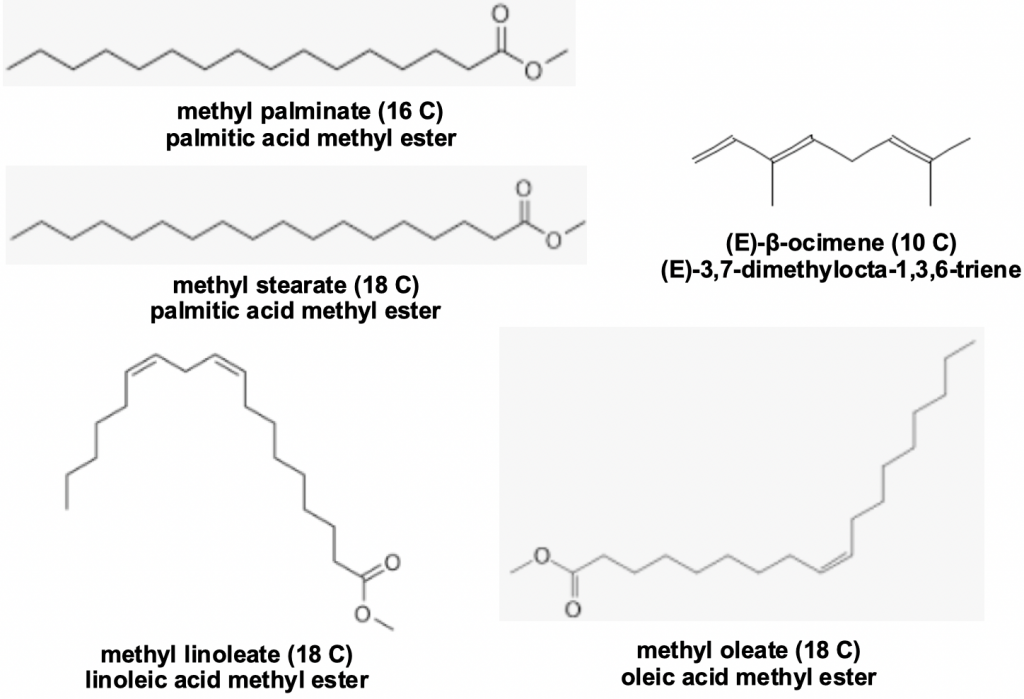
Figure 3 Brood pheromones
Worker footprint pheromone
This footprint pheromone, which is perceived olfactorily and possibly also chemotactically, is persistent but probably not colony specificxi.
Lensky and coworkers have studied the biological effect of the secretion of queen, worker and drone honey bee (Apis mellifera)i tarsal (Arnhart) glands located on the 5th segment (tarsomere) of each bee leg. They comprise a mixture of alkanes, alkenes, alcohols, organic acids, ethers, esters and aldehydes – twelve queen compounds, eleven for workers and one for dronesii. For example the appearance of queen cell cups is inhibited by queen footprint pheromone, so that crowding rather than imminent swarming may give rise to their formation. In practice I always check out lone swarm cell cups but they rarely contain eggs or larvae. The role of footprint pheromones amongst workers extends – at least in part – to marking hive entrances and flower marking.
Beyond the honey bee nest
Many honey bee pheromones appear to operate mainly within the confines of the nest, while others – augmented by the likes flower scents – have distinctive roles outside the nest. While the notion of chemical signalling being different inside as apposed to outside the nest is artificial it is nevertheless useful conceptually as many behaviours of honeybees outside the confines of the hive are important in maintaining honey bee cohesion, notably in achieving successful matings in drone congregation areas, in maintaining swarm cohesion and in allowing bees to assemble when dislocated from their hives. As we shall see there are a dazzling array of controls exercised beyond the honey bee nest as disparate as queen-drone mating behaviour and swarm behaviour, flower recognition and bee aggregation.
Readings
iBortolotti, L. and Costa, C. (2014). Chapter 5 Chemical Communication in the Honey Bee Society in Neurobiology of Chemical Communication. [Mucignat-Caretta C, editor. Boca Raton (FL): CRC Press/Taylor & Francis; 2014]. ncbi.nlm.nih.gov/books/NBK200983/#!po=9.26396 https://doi.org/10.33128/s.71.1-2.2
iiButler, C.G., Callow, R.K. and Johnston, N.C. (1962). The isolation and synthesis of queen substance, 9-oxodec-trans-2-enoic acid, a honeybee pheromone. Proceedings of the Royal Society B: Biological Sciences 155(960):417–432. doi:10.1098/rspb.1962.0009
iiiFree, J.B. (1987). Pheromones of social bees. Comstock Publishing Associates, Ithaca, NY, 218 pp.
ivSlessor, K.N., Kaminski, L.A., King, G.G.S, Borden, J.H. and Winston, M.L. (1988). Semiochemical basis for retinue response to queen honey bees. Nature 332(6162):354–56. doi:10.1038/332354a0
vBortolotti and Costa (2014) loc. cit.
viMaisonnasse, A., Alaux, C., Beslay, D., Crauser, D., Gines, C., Plettner, E. and Le Conte, Y. (2010). New insights into honey bee (Apis mellifera) pheromone communication. Is the queen mandibular pheromone alone in colony regulation? Frontiers in Zoology 7(1):18. doi:10.1186/1742-9994-7-18 https://frontiersinzoology.biomedcentral.com/articles/10.1186/1742-9994-7-18
viiKeeling, C.I., Slessor, K.N., Higo, H.A. and Winston, M.L. (2003). New components of the honey bee (Apis mellifera L.) queen retinue pheromone. Proceedings of the National Academy of Sciences 100(8):4486–4491. doi:10.1073/pnas.0836984100 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC153582/
viiiBortolotti and Costa loc. cit.
ixPeso. M. and Barron, A.B. (2014). The effects of brood ester pheromone on foraging behaviour and colony growth in apicultural settings. Apidologie 45(5):529–536.
Arnold, G., Le Conte, Y., Trouiller, J., Hervet, H., Chappe, B. and Masson, C. (1994). Inhibition of worker honeybee ovaries development by a mixture of fatty acid esters from larvae. Comptes Rendus de l’Academie des Sciences, Serie III: Sciences de la Vie 317(6):511-515. https://hal.inrae.fr/hal-02713615
Oldroyd, B.P., Wossler, T.C. and Ratnieks, F.L.W. (Sep 2001). Regulation of ovary activation in worker honey-bees (Apis mellifera): larval signal production and adult response thresholds differ between anarchistic and wild-type bees. Behavioral Ecology and Sociobiology 50(4):366-370. https://www.jstor.org/stable/4601977 doi.org/10.1007/s002650100369
xMaisonnasse, A., Lenoir, J.-C., Beslay, D., Crauser, D. and Le Conte, Y. (2010). E-β-ocimene, a volatile brood pheromone involved in social regulation in the honey bee colony (Apis mellifera). PLoS ONE, 5(10):e13531. doi:10.1371/journal.pone.00135
xiCollison, C. (23 October 2015). A closer look: tarsal glands / footprint pheromone. Bee Culture https://www.beeculture.com/a-closer-look-tarsal-glands-footprint-pheromone/
xii Collison, C. (October 2015). A closer look: Tarsal glands / footprint pheromone. Bee Culture. https://www.beeculture.com/a-closer-look-tarsal-glands-footprint-pheromone/
Lensky, Y. and Seifert, H. (1980). The effect of volume, ventilation and overheating of bee colonies on the construction of swarming queen cups and cells. Physiology Part A: Comparative Biochemistry and Physiology 67(1):97-101. doi:10.1016/0300-9629(80)90413-2
Lensky, Y. and Slabezki, Y. (1981). The inhibiting effect of the queen bee (Apis mellifera L.) foot-print pheromone on the construction of swarming queen cups. Journal of Insect Physiology 27(5):313-323. https://doi.org/10.1016/0022-1910(81)90077-9
Lensky, Y., Cassier, P., Finkel, A., Teeshbee, A., Schlesinger, R., Delorme-Joulie, C. and Levinsohn, M. (1984). The tarsal glands of honeybee queens, workers and drones. II. Biological effect. [Les glandes tarsales de l’abeille mellifique (Apis mellifera L.) reins, ouvières et faux-bourdons (Hymenoptera, Apidae). II Rôle biologique] Annales des Sciences Naturelles, Zoologie et Biologie Animale, Paris 6(13):167-175.
Lensky, Y., Finkel, A., Cassier, P., Teeshbee, A. and Schlesinger, R. (1987). The tarsal glands of the honeybee Apis mellifera L., queens, workers and drones — chemical characterization of footprint secretions. Honeybee Science 8(3):97-102. Eureka Magazine Accession: 001717070 https://eurekamag.com/research/001/717/001717070.php
xiiiSeeley, T.D. (2010). Honeybee Democracy, 280 pp. Princeton University Press.
