
The facultative eusocial carpenter bee, Exoneurella tridentata.
Alan Wade
Until recently it was widely supposed that carpenter bees, though possessing exceptionally rich and variable social bee taxa, had no eusocial species. There is new evidence that a Madagascan bee Hasinamelissa minuta and Exoneurella tridentata, from inland southern Australia (South Australia and Western Australia), both from the Allodapini tribe are indeed eusocial. The tribe Ceratinini also sports one known eusocial species, Ceratina japonica, two other species of Ceratina being all but eusocial. Like the sweat bees in the family Halictidae, the Xylocopinae carpenter bees have provided rich clues as to the origins of eusociality.
Family Apidae: Apid bees
Sub family Xylocopinae: Carpenter bees
The carpenter bee sub family Xylocopinae and its sister sub family Apinae (with its advanced eusocial corbiculate bees) make up the family Apidae. The xylocopine carpenter bees display remarkably diverse social behaviour, making them worthy subjects for the study of social evolution. They originated from a single progenitor, separate to that of the apine corbiculates.
Carpenter bees, as the name suggests, nest almost exclusively in wood, in reeds or in hollowed out stems of plants such as those of Lantana and Hydrangea. These bees are of global distribution and are comprised of four tribes, three of which display remarkably variable social behaviours: the large carpenter bees (tribe Xylocopini), the unrelated small carpenter bees (tribe Ceratinini), the allodapine bees (tribe Allodapini), and the relictual genus Manuelia (tribe Manueliini). The latter has only three species, all solitary, found solely in Chile and Argentina.
In a review of the eusociality amongst carpenter bees Rehan, Leys, Swartz and Amdami observe that:
…in the large bee subfamily Xylocopinae, a simple form of sociality was present in the ancestral lineage and there have been at least four reversions to purely solitary nesting. The ancestral form of sociality did not involve morphological worker castes and maximum colony sizes were very small. True worker castes, entailing a life-time commitment to non reproductive roles, have evolved only twice, and only one of these resulted in discrete queen-worker morphologies. Our results indicate extremely high barriers to the evolution of eusociality.
They further go on to indicate that for the Xylocopinae:
Its origins are likely to have required very unusual life history and ecological circumstances, rather than the amount of time that selection can operate on more simple forms of sociality.
Their scan of the social tribes and their taxa (Figure 3.1) point to a large number of social species including several of Australian origin including Brevineura elongataii and the endemic genus Exoneurella.
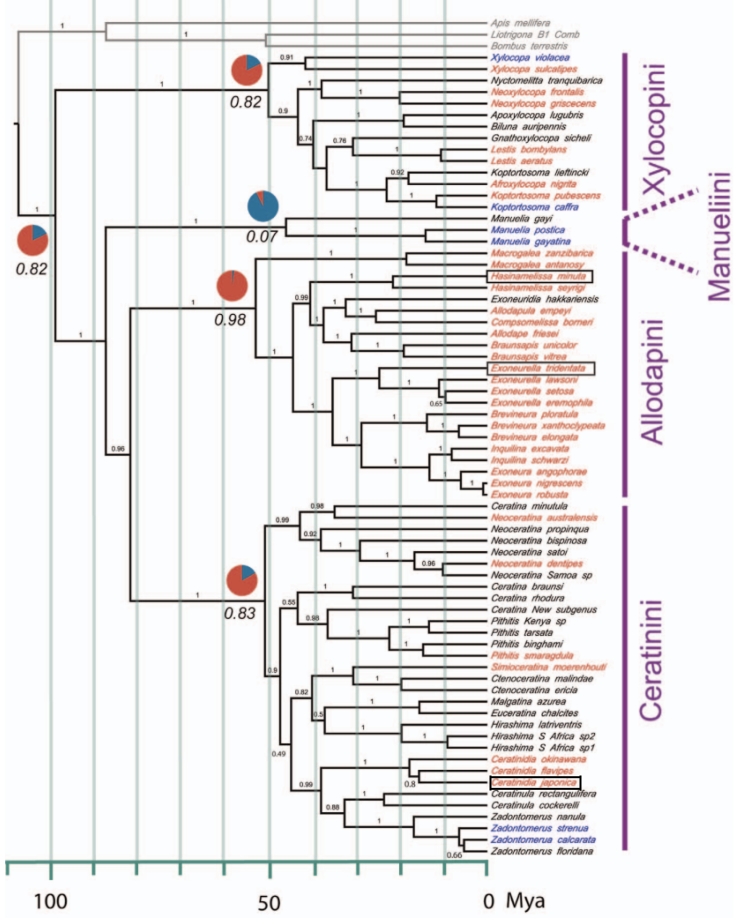
Figure 3.1 Xylocopinae sweat bee social taxa.
Xylocopine Carpenter bees: solitary species blue, social species red, unknown black.
Only the Australian Exoneurella tridentata, the Madagascan Hasinamelissa minuta and the Japanese small carpenter bee Ceratina japonica are known to be eusocial.
Tribe Xylocopini – Carpenter bees
The Xylocopini tribe with a solitary genus Xylocopa contains around 500 species, some of which have an interesting but basic social structure, Wikipedia incorrectly attributing some of them primitively eusocial status.iii
Let’s start with Rowland Stark’s Israeli study of the behaviour of Xylocopa sulcatipesi.iv The queen constructs a linear nest in a hollow plant stem sequentially provisioning each cell with pollen and nectar into each of which she lays an egg which she then seals. When the first daughter emerges she joins the queen as a guard bee while further emerging adults leave the colony. From this early start, a social colony of mother and daughter maintains and expands the colony and may develop as many as ten cells over the season. If the dominant member of this duo queen colony dies the colony transitions to become a sister-sister colony (again one dominant), or if the queen loses her nest mate she may adopt an unrelated subordinate female. While the non laying female would appear to be purely altruistic and achieve no reproductive advantage, she may take over the colony if the queen is lost. This ‘two is better than one’ plan appears to enhance the reproductive success of this bee making the social dynamic intriguing.
Stark refers to three other social species, Xylocopa aeratus, Xylocopa bombylans and Xylocopa pubescens and refers to listing of a further thirteen Xylocopini tribe species provided by Vicidomini:v Xylocopa artifex, Xylocopa carinata, Xylocopa combusta, Xylocopa fenestrata, Xylocopa frontalis, Xylocopa grisescens, Xylocopa inconstans, Xylocopa nogueirai, Xylocopa ruficeps, Xylocopa sonorina, Xylocopa suspecta, Xylocopa tranquebarorum and Xylocopa virginica with known social nesting behaviour.
Of these, the Australian green carpenter bees Xylocopa (Lestis) aeratus Smith and Xylocopa (Lestis) bombylans Fabricius (Figure 3.2) are facultatively social and have a remarkably flexible life systems.vi They have a wide distribution ranging from Kangaroo Island in South Australia to Yamba on the northern coast of New South Wales. Their nests can be multi-queened – seasonally variable in number – and, depending on natural nest hollow architecture can be linear or branched. Egg laying and brood raising strategy is influenced by many factors: nest structure, resources, climate and seasonal conditions as well as by other factors such as the need for defence. For example focus can be placed on larvae survival rather than on maximising the number of cells produced. Overall these two often sympatric species can be either solitary or social largely dependent on extraneous factors such as the length of the season. Both species are casteless and many factors such as nest cavity size dictate colony longevity.

Figure 3.2 Australian social xylocopine bees
(a) Xylocopa auratus; and
(b) Xylocopa bombylans.vii
Tribe Ceratinini – Small carpenter bees
The tribe Ceratinini is represented by the single cosmopolitan genus Ceratina.viii It contains between two and three hundred – depending on which authority one refers to – stem-nesting species and of which there is a single long-lived Australian species, Ceratina australensis (Figure 3.3).ix Its habitat stretches from New South Wales and Queensland to Papua New Guinea. It is facultatively socialx forming a colony of just two females, one a dominant reproductive and the other a subordinate queen, a nest guard. As Rehan, Richards and Swartz have observed:
Social colonies of Ceratina australensis contain only two females, and our data indicate that one female takes on both foraging and reproductive behaviour, while the second female has reduced ovarian development and wing wear suggesting neither reproduction nor foraging activity. This suggests that the reproductive female will only tolerate the presence of a nestmate if that nestmate is non-reproductive, but the non-reproductive female does not seem to take on any foraging duties. We therefore need to ask why a non reproductive female is tolerated, and why that female should forego reproduction to remain as a non-reproductive, non-foraging nestmate. The social primary may tolerate the secondary female at the natal nest without contributing foraging effort, as the mere presence of the secondary might contribute to the colony by guarding brood while the primary reproductive is away from the nest. In addition, the social secondary may be a hopeful reproductive waiting to inherit the nest site from the social primary.

Figure 3.3 Australian social ceratinine bee Ceratina australiensis.xi
They also note that this arrangement is replicated in other Xylocopa taxa:
This situation arises in social nests of some Xylocopa species in which the dominant female is both the primary forager and the primary reproductive while the secondary female remains at the nest acting as a guard waiting for nest inheritance and supersedure.
The Ceratina genus is truely polymorphic: Sandra Rehan distils this elegantly:xii
Ceratina are stem-nesting bees with a broad range of social behavior from solitary and subsocial to semisocial and eusocial colony organization. As in other xylocopine bees, the adult life span of Ceratina is unusually long, a life history trait that strongly influences their social behavior.
Illustrative of this is the facultatively eusocial Ceratina japonica. Fairly typically for this genus, this small carpenter bee nests in pithy stems of plants producing about eight offspring per year. It is widespread in Japan and both solitary and eusocial colonies are found in sympatry, that is in the same locality. Shell and Rehanxiii outline the genetic basis for these alternative lifestyles:
This ontogenetic split is highly consequential, as queens and workers go on to exhibit substantially different physiologies and behavioural states. While a queen spends most of her life inside the nest, producing brood and interacting with her colony, her worker offspring perform a variety of complex, age-related tasks to support and protect the nest.
This interpretation aligns very closely with Paul Hurd’s stated mechanism for production of queens and worker bees amongst honey bees.xiv
In solitary nests of Ceratina japonica the queen undertakes all the duties of raising brood in the same manner as any purely solitary species does. However in social nests, comprising two adult females, the larger and reproductively dominant guards the nest while the smaller daughter forages. Since the duo team can persist for two or more years, Ceratina japonica is facultatively eusocial, though rather primitively so.
Another species, Ceratina calcarata, displays very near eusocial behaviour.xv The queen is long-lived and tends overlapping generations of brood. Further the queen produces dwarf elder daughters as well as regular daughters. The regular daughters do not forage and stay in the nest over winter departing to establish new nests as solitary foundresses in spring. The elder dwarf daughters emerge early in autumn and forage and feed their larger sisters but die over winter and are never reproductive. So while these bees tend adult siblings, they do not live to nurture the next generation of bees, though the overwintering queen will produce more offspring after winter. Most of the conditions of eusociality are met but not the critical worker fostering facility of tending the next generation.
Rehan and coworkersxvi signal that Ceratina calcarata:
…is neither solitary nor eusocial but truly at the brink of eusociality with overlapping generations and reproductive division of labour, but no cooperative brood care.
The social behaviour in Ceratina is also amplified not only by typical longevity of many species but also in their mating behaviour. So while Ceratina australensis, distributed widely in south eastern Australia, is monandrous, multiple mating (polyandry) is also common in the genus, as it is among honey bees. Mikát and coworkers describe the behaviour of Ceratina nigrolabiata notably the role of male bees in performing nest guard duties (Figure 3.4).xvii
Mikát and other coworkers undertook a study of the life histories of the seven of the eight Mediterranean Ceratina species found in Cyprus, five of which formed multi queen nests (Table 3.1), in part conducted to complement what is known about the better studied species in Japan and North America. As a rule they found that social nests did not produce more brood than equivalent single queen colonies but multi queen colonies were more likely to survive and hence were more likely to be reproductively successful. Their study encompassed a wide-ranging review of the importance of the tribe Ceratinini (monophyletic as Ceratina) drawing in also the Xylocopini and Allodapini tribes (that together make up the carpenter bee Xylocopinae sub family) and the sometimes social orchid bee Euglossini tribe (in the corbiculate Apinae sub family) in assessing the origins of obligate eusociality found amongst honey, stingless and bumble bees and amongst some wasps.
| Species | Number of nests | Nesting status | ||||
| Solitary | Multi female | Orphaned | Male and female | Dead | ||
| Ceratina bispinosa | 11 | 8 | 3 | – | – | – |
| Ceratina cypriaca | 164 | 148 | 10 | 3 | 2 | 1 |
| Ceratina dallaoreana | 65 | 56 | – | 9 | – | – |
| Ceratina chrysomalla | 77 | 63 | 2 | 12 | – | – |
| Ceratina mandibularis | 115 | 88 | 20 | 4 | 2 | 1 |
| Ceratina parvula | 67 | 61 | 4 | 2 | – | – |
| Ceratina schwarzi | 14 | 14 | – | – | – | – |
Table 3.1 Cyprian Ceratina nest dynamics after Mikát and coworkers.xviii
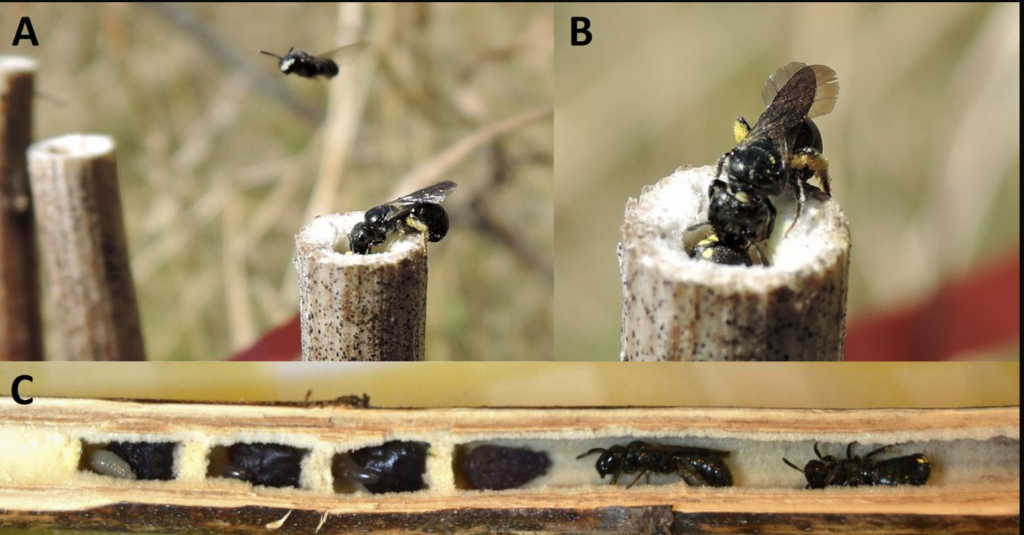
Figure 3.4 Ceratina nigrolabiata nesting behaviour:
(A) female bee returning with a pollen load, non-nest male hovering above nests: males regularly change nests;
(B) female scratching guarding male, marked yellow, to gain nest entry; and
(C) opened brood nest with showing completed brood cells and cell provisioning, both female (left) and male (right) in burrow.
Tribe Allodapini – Allodapine carpenter bees
The small allodapine carpenter bees of the tribe Allodapini are found in sub-Saharan Africa, South East Asia, and Australasia.xix Many of the species of this tribe form small social colonies comprising a group, often two, of females raising brood cooperatively.xx Only two species have been identified as containing a functionally sterile worker caste, one in the Australian genus Exoneurella (E. tridentata), the other a Madagascan bee in the recently reassigned genus Hasinamelissa of which more of both below.
Of the fifteen allodapine genera, seven genera are found in Australia, but only one of these, Exoneurella,xxi is endemic to Australia. It comprises just four species: Exoneurella tridentata, Exoneurella setosa, Exoneurella eremophilaxxii and Exoneurella lawsoni, all nesting in reeds or twigs and all forming small colonies containing two to (occasionally) eight females. Of these Exoneurella tridentata is facultatively eusocial while the others are solitary or facultatively social. Exoneurella tridentata in found in extensive tracts across semi arid South Australia and south western Western Australia. As Dew and Swartz note:xxiii
This species [Exoneurella tridentata] is unique among the allodapines in that it is the only species to demonstrate the most extreme form of insect sociality, eusociality, which involves large colony sizes with distinct queen and worker castes. Queens in this species are much larger than workers, with widened head and mandibles and greatly enlarged and distally splayed abdomens (Figure 3.5). The species may form colonies of up to sixty or more individuals, far greater than any other allodapine bee.xxiv Exoneurella tridentata is important for two reasons. Firstly, it provides an opportunity to understand how complex sociality can evolve from simple forms of sociality. Secondly it is one of the few native bee species in South Australia whose foraging activity extends throughout the year…
Note: The allodapine Hasinamelissa minuta discussed below is now also kwown to be eusocial.
The southeastern Australian allodapine, Exoneura robustaxxv comes close to meeting the criteria for a facultatively eusocial species, being long lived, exhibiting a range of social expressions, and having subordinate (non laying) queens who assist in brood rearing over a couple of seasons but which nevertheless do not have a well-defined worker caste.

Figure 3.5 Exoneurella tridentata ‘castes’:
(a) queen; and
(b) worker.xxvi
Dew and coworkers describe the nest and social structure of the casteless Exoneurella setosa (Figure 3.6).xxvii It illustrates a lack of ovarian skew among many allodapine bees and the evolution of casteless social behaviour in many species:
Exoneurella setosa, which forms social colonies… lack reproductive hierarchies and are therefore ‘casteless’; an intriguing discovery given that a congeneric eusocial species exhibits the greatest morphological distinction between queen and worker castes in the entire subfamily Xylocopinae (Apidae). Exoneurella setosa exhibited a modal colony size of two females per nest and we analysed nest-mate differentiation in ovarian development, body size and wing wear (a proxy for foraging activity). We… ascertain[ed] that multi female nests lack evidence for reproductive skew… suggest[ing] that nest-site limitation is a key driver for eusocial organisation within the Xylocopinae and that the absence of such environmental limitations, combined with minor benefits for group nesting, can select for casteless social organisation.

Figure 3.6 Australian social allodapine bee Exoneura robusta.xxviii
A further species, the Madagascan Hasinamelissa minuta (syn Halterapis minuta), is clearly eusocialxxix though strangely so. Colony numbers do not total more than six adult females with up to about twelve larvae at different stages of development:
This species has a unique form of brood provisioning, where a clutch of eggs is mass provisioned with a single, long cylindrical pollen mass (Figure 3.7), and larvae gradually eat their way along this food store. Most colonies contained more than one adult female, with generational overlap and very strong size-related ovarian differentiation among nest mates, indicating the the species is eusocial. Sex allocation was extremely female biased, and this is probably linked to reproductive skew within colonies.
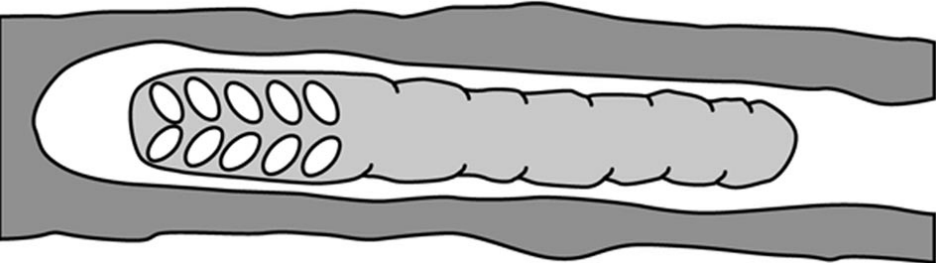
Figure 3.7 Madagascan allodapini bee Hasinamelissa minuta mass provisioned brood nest.
The social lives of other Madagascan allodapine bees have been poorly studied and may reveal other unexpected life systems.xxx Navigating the difficult path to evolution of eusocial taxa amongst the allodapine tribe is summarised aptly by Schwarz, Tierney, Rehan, Chenoweth, and Cooper:xxxi
…in the bee tribe Allodapini, the earliest societies did not entail a foraging worker caste, but instead comprised females sharing a nest with supersedure of dominance. Subordinates delayed foraging until they became reproductively active, whereupon they provided food for their own brood as well as for those of previously dominant females.
In retrospect and prospect
Overall we have observed that bees within both the sweat and now the carpenter bee lineages have experimented widely to form social and eusocial communities. As such they have played dice with Darwinian social evolution on a grand scale. The carpenter bees have distinctively limited eusociality illustrating the severe constraints imposed of development of this trait across the tribe despite the fact that they are closely related to the commonly eusocial corbiculates in the same sub family.
The most advanced corbiculate bees – and many of the predatory eusocial vespid wasps and all the ants – have taken eusociality to its limit, generating massive numbers of reproductively sterile offspring to bolster their numbers, their harvesting power, and their ecological dominance. We now have some clues as to how they navigated the transition from solitary to several forms of eusocial colony organisation.
In the concluding parts on corbiculate bees, we will return to where we started revisiting the Apinae tribes: the Apini (honey bees), the Meliponini (the stingless bees), the Bombini (the bumble bees) and the Euglossini (the sometimes social, but mainly solitary orchid bees). These tribes all have very different stories to tell, though the taxonomists assure us that, in the very distant past, the corbiculate bees also had but one common primitively eusocial progenitor.
Readings
iRehan, S.M., Leys, R., Schwarz, M.P. and Amdam, G.V. (2012). A Mid-Cretaceous origin of sociality in xylocopine bees with only two origins of true worker castes indicates severe barriers to eusociality. PLoS ONE 7(4):e34690–. doi:10.1371/journal.pone.0034690
Michener, C.D. (1990). Castes in xylocopine bees. In Engels, W. (ed). Social insects: an evolutionary approach to castes and reproduction. pp.123-146. Berlin, Heidelberg: Springer Berlin Heidelberg. https://link.springer.com/chapter/10.1007/978-3-642-74490-7_7 doi:10.1007/978-3-642-74490-7_7
iiJoyce, N.C. and Schwarz, M.P. (2006). Sociality in the Australian allodapine bee Brevineura elongata: Small colony sizes despite large benefits to group living. Journal of Insect Behavior 19(1):45-61. doi:10.1007/s10905-005-9004-1 Rayment, T. (1954). New bees and wasps—Part XXII. The altruistic reed-bees, Exoneura. Victorian Naturalist 71(1):13-16. https://archive.org/details/biostor-246173 https://www.biodiversitylibrary.org/item/127313#page/15/mode/1up
iiiWikipedia (accessed 26 June 2023). Carpenter bee. https://en.m.wikipedia.org/wiki/Carpenter_bee
ivStark, R.E. (1992). Cooperative nesting in the multivoltine large carpenter bee Xylocopa sulcatipes Maa (Apoidea: Anthophoridae): Do helpers gain or lose to solitary females? Ethology 91(4):301-310.
doi:10.1111/j.1439-0310.1992.tb00871.x
vVicidomini, S. (1996). Biology of Xylocopa violacea (Hymenoptera): In-nest ethology. Italian Journal of Zoology63(3):237-242. https://www.tandfonline.com/doi/pdf/10.1080/11250009609356139 doi:10.1080/11250009609356139
viSteen, Z. and Schwarz, M.P. (2000). Nesting and life cycle of the Australian green carpenter bees Xylocopa (Lestis) aeratus Smith and Xylocopa (Lestis) bombylans (Fabricius) (Hymenoptera: Apidae: Xylocopinae). Australian Journal of Entomology 39(4):291-300. doi:10.1046/j.1440-6055.2000.00195.x
viiiNaturalist (accessed 18 October 2023). Xylocopa (Lestis) aeratus. https://canberra.naturemapr.org/sightings/4206805
Atlas of Living Australia (accessed 18 October 2023). Xylocopa (Lestis) bombylans https://images.ala.org.au//image/f7d29be4-286d-46e2-86ad-5186b5033037
viiiWikipedia (accessed 24 June 2023). Ceratina. https://en.m.wikipedia.org/wiki/Ceratina
ixMichener, C.D. (1962). The genus Ceratina in Australia, with notes on its nests (Hymenoptera: Apoidea). Journal of the Kansas Entomological Society 35(4):414-421. http://www.jstor.org/stable/25083289
xOppenheimer, R.L., Shell, W.A. and Rehan, S.M. (2018). Phylogeography and population genetics of the Australian small carpenter bee, Ceratina australensis. Biological Journal of the Linnean Society 124(4):744-755. doi:10.1093/biolinnean/bly070 https://academic.oup.com/biolinnean/article/124/4/747/5033982?login=false
Rehan, S.M., Richards, M.H. and Schwarz, M.P. (2010). Social polymorphism in the Australian small carpenter bee, Ceratina (Neoceratina) australensis. Insectes Sociaux 57(4):403-412. doi:10.1007/s00040-010-0097-y
xiAtlas of Living Australia (accessed 16 October 2023). Ceratina (Neoceratina) australensis (Perkins, 1912). https://bie.ala.org.au/species/https://biodiversity.org.au/afd/taxa/017eefeb-5c5f-4b1b-bf48-868166b4eea0
xiiRehan, S.M. (2020). Small carpenter bees (Ceratina). In Starr, C.K. (ed). Encyclopedia of Social Insects. Springer, Cham. https://link.springer.com/referenceworkentrarr, C.K (y/10.1007/978-3-319-90306-4_106-1
xiiiShell, W.A. and Rehan, S.M. (2022). Social divergence: Molecular pathways underlying castes and longevity in a facultatively eusocial small carpenter bee. Proceedings of the Royal Society B 289(1971):20212663.
https://royalsocietypublishing.org/doi/10.1098/rspb.2021.2663 https://royalsocietypublishing.org/doi/epdf/10.1098/rspb.2021.2663
xivHurd, P. (22 August 2018). Epigenetic patterns determine if honeybee larvae become queens or workers. Queen Mary University of London. https://www.qmul.ac.uk/media/news/2018/se/epigenetic-patterns- determine-if-honeybee-larvae-become-queens-or-workers-.html
xvRehan, S.M., Glastad, K.M., Lawson, S.P. and Hunt, B.G. (2016). The genome and methylome of a subsocial small carpenter bee, Ceratina calcarata. Genome Biology and Evolution 8(5):1401-1410. doi:10.1093/gbe/evw079
Rehan, S.M., Berens, A.J. and Toth, A.L. (2014). At the brink of eusociality: transcriptomic correlates of worker behavior in a small carpenter bee. BMC Evolutionary Biology 14(1):260-270. doi:10.1186/s12862-014-0260-6 https://bmcecolevol.biomedcentral.com/articles/10.1186/s12862-014-0260-6
Rehan, S.M. and Richards, M.H. (2010). Nesting biology and subsociality of Ceratina calcarata (Hymenoptera: Apidae). Canadian Entomologist 142(1):65-74. http://www.rehanlab.com/uploads/2/1/4/3/21434988/rehan_&_Richards_2010_-_ceratina_calcarata_nesting_biology.pdf
Rehan, S.M. and Richards, M.H. (2010). The influence of maternal quality on brood sex allocation in the small carpenter bee, Ceratina calcarata. Ethology 116(9):876-887. doi:10.1111/j.1439-0310.2010.01804.x
xviRehan, Berens and Toth (2014) loc. cit.
xviiMikát, M., Janošík, L., Černá, K., Matoušková, E., Hadrava, J., Bureš, V. and Straka, J. (2019). Polyandrous bee provides extended offspring care biparentally as an alternative to monandry based eusociality. Proceedings of the National Academy of Sciences of the United States of America 116(13):201810092. doi:10.1073/pnas.1810092116 https://sci-hub.mksa.top/10.1073/pnas.1810092116
Mikát, M., Fraňková, T., Benda, D. and Straka, J. (2022). Evidence of sociality in European small carpenter bees (Ceratina). Apidologie 53(2):18. https://doi.org/10.1007/s13592-022-00931-8
xviiiMikát, Fraňková, Benda and Straka (2022) loc. cit.
xixWikipedia (accessed 28 June 2023). Allodapini. https://en.m.wikipedia.org/wiki/Allodapii
Michener, C.D. (2007). The Bees of the World, second edition. The Johns Hopkins University Press, Baltimore, Maryland. Tribe Allodapini. Section 90. pp.619-632.
xxJoyce, N.C. and Schwarz, M.P. (2006). Sociality in the Australian allodapine bee Brevineura elongata: Small colony sizes despite large benefits to group living. Journal of Insect Behavior 19(1):45-61. doi:10.1007/s10905-005-9004-1
xxiDew, R.M., Stevens, M.I. and Schwarz, M.P. (2018). Taxonomy of the Australian allodapine bee genus Exoneurella (Apidae: Xylocopinae: Allodapini) and description of a new Exoneurella species. Insect Systematics and Diversity 2(1):ixx013. doi:10.1093/isd/ixx013 https://academic.oup.com/isd/article/2/1/ixx013/4842112?login=false
Reyes, S.G., Cooper, S.J.B. and Schwarz, M.P. (1999). Species phylogeny of the bee genus Exoneurella Michener (Hymenoptera: Apidae: Allodapini): Evidence from molecular and morphological data sets. Annals of the Entomological Society of America 92(1):20-29. doi:10.1093/aesa/92.1.20
https://academic.oup.com/aesa/article/92/1/20/22170?login=false
xxiiDew, R., Tierney, S., Gardner, M. and Schwarz, M. (2017). Casteless behaviour in social groups of the bee Exoneurella eremophila. Apidologie 49(2):265-275. doi:10.1007/s13592-017-0550-2 https://link.springer.com/content/pdf/10.1007/s13592-017-0550-2.pdf
xxiiiDew, R.M. and Schwarz, M.P. (2013). Distribution of the native South Australian bee Exoneurella tridentata in Western Myall (Acacia papyrocarpa) woodlands. The South Australian Naturalist 87(2):70-74.
https://www.researchgate.net/publication/264348692_Distribution_of_the_native_South_Australian_bee_Exoneurella_tridentata_in_Western_Myall_Acacia_papyrocarpa_woodlands
Houston, T.F. (1977). Nesting biology of three allodapine bees in the subgenus Exoneurella Michener (Hymenoptera: Anthophoridae). Transactions of the Royal Society of South Australia 101(4):99-113. https://www.biodiversitylibrary.org/partpdf/271296
https://archive.org/details/biostor-245849
Hurst, P.S. (2001). Social biology of Exoneurella tridentata, an allodapine with morphological castes and perennial colonies. PhD Thesis, The Flinders University of South Australia, Adelaide, Australia. Unpublished.
xxivDew, R.M., Rehan, S.M., Tierney, S.M., Chenoweth, L.B. and Schwarz, M.P. (2012). A single origin of large colony size in allodapine bees suggests a threshold event among 50 million years of evolutionary tinkering. Insectes Sociaux 59(2):207-214. doi:10.1007/s00040-011-0206-6
xxvLanger, P., Hogendoorn, K., Schwarz, M.P. and Keller, L. (2006). Reproductive skew in the Australian allodapine bee Exoneura robusta. Animal Behaviour 71(1):193-201. https://serval.unil.ch/resource/serval:BIB_2359B5E165CA.P001/REF.pdf
xxviSteen, Z. and Schwarz, M.P. (1998). Within-nest behaviour in a eusocial Australian allodapine bee Exoneura (Exoneurella) tridentata Houston (apidae xylocopinae). Transactions of the Royal Society of South Australia 122(1-2):55-63. https://www.biodiversitylibrary.org/page/41319937#page/62/mode/1up
Schwarz, M.P. and Tierney, S.M. (2000). Allodapine bees. pp.22-27. In Starr, C.K. (ed). Encyclopedia of Social Insects. https://sci-hub.mksa.top/10.1007/978-3-030-28102-1_117 doi:10.1007/978-3-030-28102-1_11
xxviiDew, R.M., Tierney, S.M. and Schwarz, M.P., (2018). Lack of ovarian skew in an allodapine bee and the evolution of casteless social behaviour. Ethology, Ecology and Evolution 30(1):51-69. https://www.tandfonline.com/doi/abs/10.1080/03949370.2017.1313784
xxviiiAtlas of Living Australia (accessed 16 October 2023). Exoneura (Exoneura) robusta Cockerell, 1922. https://bie.ala.org.au/species/https://biodiversity.org.au/afd/taxa/1bbac329-5da5-4f71-a34e-efdc3153ba52#gallery
xxixSchwarz, M.P., Tierney, S.M., Rehan, S.M., Chenoweth, L.B. and Cooper, S.J. (2011). The evolution of eusociality in allodapine bees: workers began by waiting. Biology Letters 7(2):277-280. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3061166/ https://royalsocietypublishing.org/doi/abs/10.1098/rsbl.2010.0757
xxxChenoweth, L.B., Fuller, S., Tierney, S.M., Park, Y.C. and Schwarz, M.P. (2008). Hasinamelissa: a new genus of allodapine bee from Madagascar revealed by larval morphology and DNA sequence data. Systematic Entomology33(4):700-710. doi.org/10.1111/j.1365-3113.2008.00432.x https://www.academia.edu/20674514/Hasinamelissa_a_new_genus_of_allodapine_bee_from_Madagascar_revealed_by_larval_morphology_and_DNA_sequence_data
Schwarz, M., Tierney, S., Zammit, J., Schwarz, M. and Fuller, S. (2005). Brood provisioning and colony composition of a Malagasy species of Halterapis: Implications for social evolution in the allodapine bees (Hymenoptera: Apidae: Xylocopinae). Annals of The Entomological Society of America 98(1):126-133. doi:10.1603/0013-8746(2005)098[0126:BPACCO]2.0.CO;2 https://fac.flinders.edu.au/dspace/api/core/bitstreams/22078452-7513-49d7-9e88-626a2aa53dc7/content https://academic.oup.com/aesa/article-abstract/98/1/126/161149?login=false
xxxiSchwarz, Tierney, Rehan, Chenoweth and Cooper (2011) loc. cit.
Schwarz, M.P., Bull, N.J. and Hogendoorn, K. (1998). Evolution of sociality in the allodapine bees: A review of sex allocation, ecology and evolution. Insectes Sociaux 45(4):349-368. doi.org/10.1007/s000400050095
